17B14: Exam Report
Explain the mechanisms responsible for the cell resting membrane potential (60% of marks) and describe the Gibbs Donnan Effect (40% of marks).
35% of candidates passed this question.
A good answer included a definition of the resting membrane potential and a clear description of the factors that determine it. Explanation of these factors should have included a detailed description of the selective permeability of the membrane, electrochemical gradients and active transport mechanisms. Answers should demonstrate awareness of the Nernst equation and the Goldman-Hodgkin-Katz equation. These were often confused, sometimes with the Gibbs-Donnan effect. Descriptions of the Gibbs-Donnan effect generally lacked detail and understanding. The better answers included a definition and discussed in detail the influence of non-diffusible ions (intracellular proteins) on the distribution of diffusible ions.
Eii / 17B14: Explain the mechanisms responsible for the cell resting membrane potential (60 marks) and describe the Gibbs Donnan effect (40 marks)
Definition
The RMP is the electrical potential across a plasma membrane when the cell is in a non-excited state
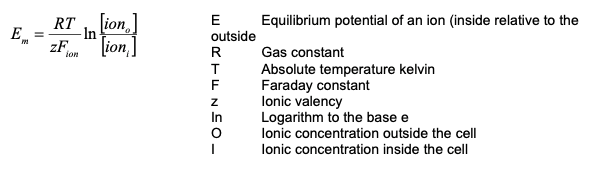
Equilibrium Potential
Nernst Equation
- The Nernst Potential (for each ion) calculates the potential difference the ion would produce if the membrane was permeable to it
Ion
K
[Intracellular] mM
150
[Extraceullular] mM
5
Nernst Potential (mV)
-90
Ion
Cl
[Intracellular] mM
10
[Extraceullular] mM
125
Nernst Potential (mV)
-70
Ion
Na
[Intracellular] mM
15
[Extraceullular] mM
150
Nernst Potential (mV)
+60
- At rest the Nernst potentials of Na & Cl most closely match Membrane Potential because these ions are freely diffusing, whereas Na is not
Goldman-Hodgkin-Katz Equation
- A modification of the Nernst Eqn
- Takes into account the permeability factors for each ion and constructs the total membrane resting potential
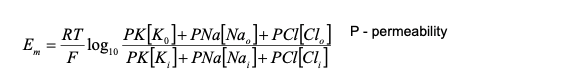
Resting Membrane Potential
- By definition the cell membrane must be semi-permeable (to create a potential)
- The cell membrane encompasses sodium (chemical gradient) and is freely permeable to K & Cl (electrochemical gradient)
- Ion channels and transport proteins regulate the movement of molecules across the membrane, allowing for an unequal distribution of charge through anions and cations
- The resulting chemical and electrical forces comprise the RMP
- The RMP varies depending on cell type
- Skeletal Muscle -90mV
- Myelinated Peripheral Nerve -70mV
- Cardiac Muscle -90mV
- Smooth Muscle -50mV
Generation of an Electro-Chemical Gradient
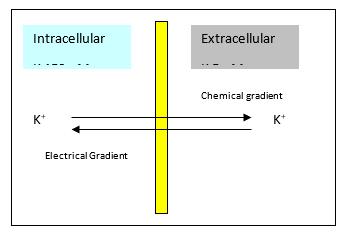
Potassium
- K ions cross the membrane through open channels
- Internal [] 150mM
- External [] 5mM
- K moves down its concentration gradient until opposing electrical gradient prevents further movement
- Results in an electrical potential
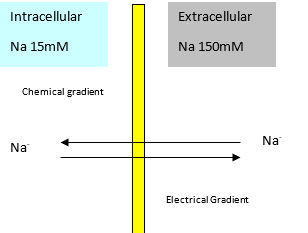
Sodium
- Sodium is not freely permeable
- Uneven concentration created by Na-K-ATPase
- Small electrochemical potential due to low membrane permeability
Chloride
- Chloride can pass freely
- Creates an electrochemical gradient
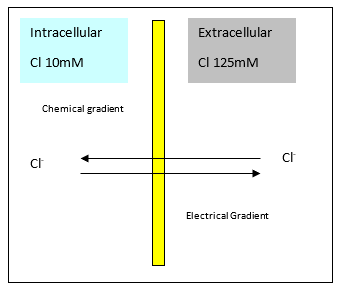
Gibbs-Donnan Effect
- Applies when one compartment contains non-diffusable ions
- Proteins which lack permeability are also contributing to the membrane potential
- The proteins inside the cell are adding a (small) negative charge to the overall membrane potential
- This accounts for the different plasma and ISF concentrations of the ions
Function of Membrane Potential
- Conduction of electrical signalling
- Excitation-Contraction-Coupling
- Electrochemical Gradients use energy to run cell machinery