F10iv: Define humidity and give an outline of the importance of humidification
Principles of Humidification
- H2O consists of molecules
- Molecules have variable kinetic energy (E)
- Temp of H2O determined by mean kinetic E of these molecules
- At liquid/gas interface, the molecules with enough E overcome attractive forces of H2O in liquid & escape into the gas as a vapour
- Molecules escaping have the most kinetic E
- When they leave, the mean kinetic E of the liquid H2O ↓ (& ∴so does temp)
- The molecules in vapour form will exert a partial pressure within the gas
- In a sealed container at constant temp., equal no’s of molecules are going liq ⮂ vapour
- At steady state the pressure being exerted ka SATURDATED VAPOUR PRESSURE
Definitions
- Latent heat of vaporisation: the heat required to convert 1g of a substance from liquid phase to gas phase at a given temp
- Humidification: the addition of water vapour to a gas
- Absolute humidity (g/m3): the mass of H2O vapour (g) present in a given volume of air (m3)
- g /m3 = mg / L
- Relative humidity (%): a ratio; of the mass of H2O vapour in a given volume of air vs. the mass required to fully saturate that volume of air
- Saturated vapour pressure (mmHg): the vapour pressure (pressure exerted) when the liquid & vapour phase are in equilibrium
Normal Values
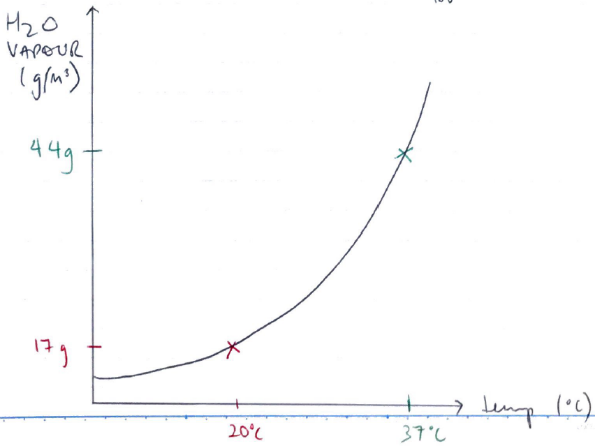
- PSVP = 44mmHg at 1ATM
- At 20°C, 1m3 of air in fully saturated with 17g H2O
- At 37°C, 1m3 of air is fully saturated with 44g H2O (i.e. at carina)
- ∴As temp ↑, relative humidity ↓
- e. 1m3 of air with 17g water @ 20°C
- At 37°C, 1m3 of air will have relative humidity (because saturated is 44g at 37°C)
- ∴↓relative humidity at ↑temp
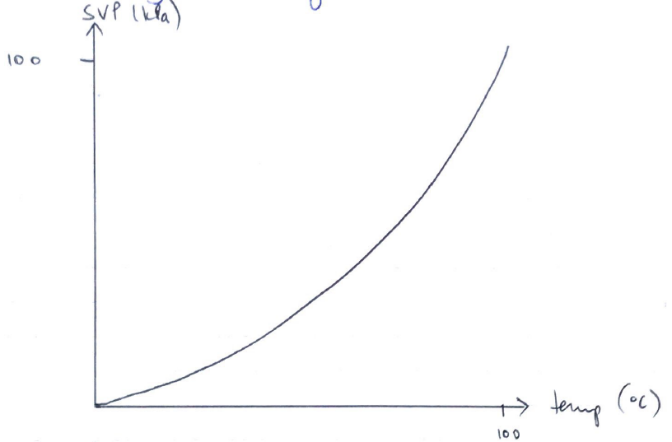
SVP depends on temp
- ↑temp. of liquid
- ↑kinetic E of molecules
- More molecules can escape the liquid phase
- Exerting a greater saturated vapour P
Physiological Humidifcation
Inspiration
- Nose breathing → nasal turbinates ↑SA of nasal epithelium
- Air is warmed by heat from radiant blood supply
- As air is warmed ↑T = ↑SVP
- Moisture on epithelium evaporates → joins dry air to ↑humidity to 90%
- NOTE: mouth breathing ↓relative humidity to ~70%
- Humidification continues in similar way down pharynx
- Just below carina is ISOTHERMIC SATURATION BOUNDARY (ISB) = anatomical part where inspired gas becomes fully saturated
→ Distal to ISB, temp. & humidity is constant
→ Proximal to ISB the anatomy is acting as a HME
- @ Carina
- Relative H = 100%
- Absolute H = 44g/m3 (37°C)
Expiration
- Expired gas transfers heat back to cooler trachea & nasal mucosa
- As gas cools it holds less vapour (↓T = ↓SVP)
- ∴Condensation occurs on nasal mucosa → some H2O reabsorbed
- ↓potential H2O loss from 300 → 150mL/day
Importance of Humidification
- ↓H2O loss
- Ciliary function
- ↓Mucous viscosity
- ↓Risk sputum plugging
- Without humidity, cilia die → tracheal epithelium becomes keratinised, ulcerated & necrosed
- Excess humidity also bad → ciliary dysfunction, APO, ↓Na+
The Ideal Humidifier
- Delivers gas at temp 32 – 36°C with water content 43g/m3
- Set temp. remains constant
- Not affected by gas flow
- Simple to service
- Humidification can be provided for air, O2 & other gases
- Can be used with SPV/controlled ventilation
- Safety → prevents overheating & electrocution
- Doesn’t affect DS, Resistance, Compliance
- Sterility uncompromised
- Cost effective
Methods & Devices in ICU
Passive: HME → no E/H2O source
Active: H2O baths, bubble humidifiers, Passover, nebulisers
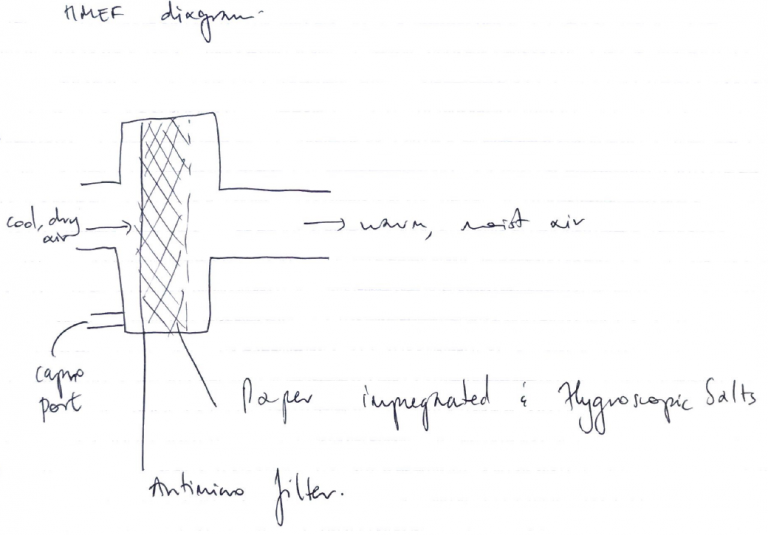
HME
- Does not require external E/H2O to function
- Plastic casing with inlet & outlet which forms a closed unit
- Material with low thermal conductivity (paper) is impregnated with hygroscopic (H2O retaining) salts i.e. calcium
- On EXPIRATION → warm, moist air passes through the device → gas is cooled → H2O condenses
- The element/matrix is warmed by the latent heat of H2O condensing on it & by exhaled gas
- On INSPIRATION → moisture retained evaporates, humidifying the inspired gas & warming the air
@OPTIMUM:
- Air reaching trachea has H2O vapour of 34g/m3
- Reaches humidity 25g/m3
- HME Relative Humidity = 60 – 70%
Advantages
- Cheap & simple
- No power
- 60 – 70% humidification
- Retains heat & moisture
- Improves ciliary function & mucous clearance
Disadvantages
- ↑DS
- R 0.1 – 2cm H2O
- Bulk
- Can become occluded
- ↓efficiency at ↑MV/VT
- Can take 10 – 20 mins to reach full efficiency (a relative humidity of 70%)
Heated H2O Bath Humidifer (Passover Humidifer)
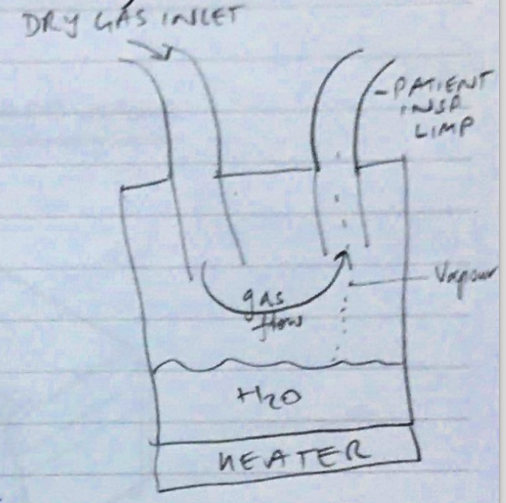
MoA
- Heater base & water filled chamber
- Water is heated → ↑T = ↑SVP
- Fresh gas passes through & collects H2O vapour
- Carries humidified gas to patient
- Insp. limb may contain a heating wire so ↓H2O loss as it goes to patient (because ↓temp along INSP LIMB)
Advantages
- Less infection risk cf aerosols because vapour cannot carry microbes
- No ↑R/DS
Disadvantages
- Condensation build up in INSP LIMB
- If thermostat fails → scaling of patient
- Bac/fungal colonisation of H2O reservoir
Bubble Humidifier
MoA
- Simplest active humidifier
- Fresh gas flow is passed through H2O reservoir
- Bubbles absorb H2O vapour as they pass to the surface of the reservoir
Advantages
- Compact
- Cheap
- ↑humidifier cf. HME
Disadvantages
- Not v. efficient
- ↑Absolute H ~20g/L
- Bac growth in bath
- H2O aerosols can transmit infection
Nebulised Humidifiers
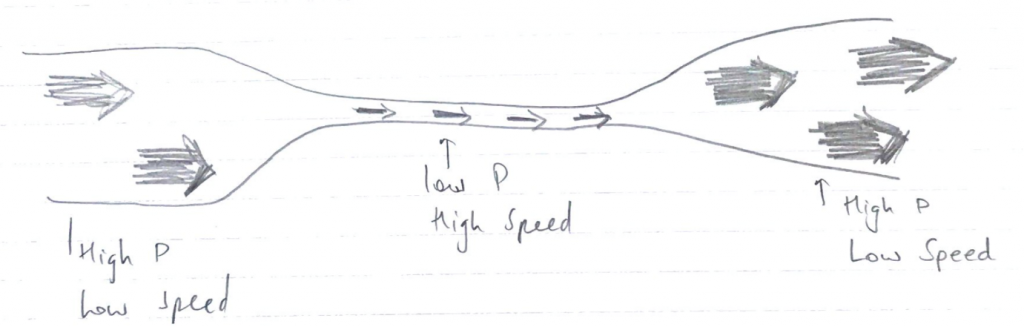
- Relies on Bernoulli Effect
- BERNOULLI: if the pressure along a tube is measured at the narrowest point of the tube, the pressure will be lower than elsewhere & often below atmosphere
- Flowing fluid contains 2 energies:
- POTENTIAL (pressure)
- KINETIC (flow)
- Law of Conservation of Energy → total E must remain constant
- Therefore, as gas move through a narrow point of tubing
- Gas Velocity increases (Kinetic E)
- Pressure decreases (Potential E)
So that the total E remains constant
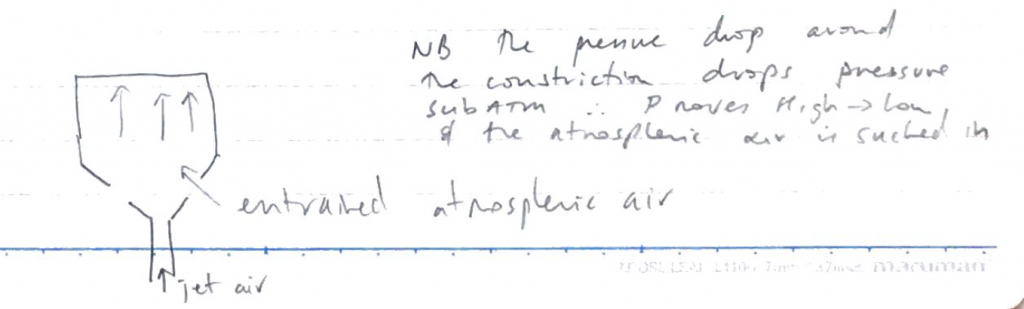
The Bernoulli effect allows entrainment of air/fluid around constricted site
Nebuliser
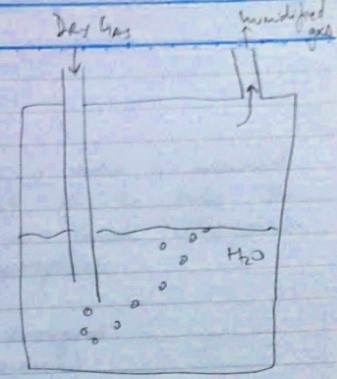
- A nebuliser is a device that converts liquid into aerosol droplets
- High velocity gas driven by the nebuliser through a tube sitting in a reservoir of H2O
- As gas jets through the narrowest part of the -ve P draws H2O into tube
- The high velocity gas breaks the H2O droplets
- May contain an ANVIL which sits in path of entrained droplets
- When liquid hits ANVIL it disperses into even smaller objects
Advantages
- ↑Humidity
- ↓R/DS
Disadvantages
- oedema
- Bac/viral route
- Expensive
- Needs sterile H2O
Droplet Size Matters
- 20 μm → nuisance → forms pools of H2O in tubing
- 5 μm → falls into trachea
- <1 μm → pass into alveoli → extremely stable ^ can be INSP/EXPIRED again (this is the size you want to deliver drugs)
ULTRASONIC → a vibration plate of 2 – 3MH2 positioned in a H2O reservoir transmits vibrations to H2O → to create H2O droplets
PRX & Wet Bulb
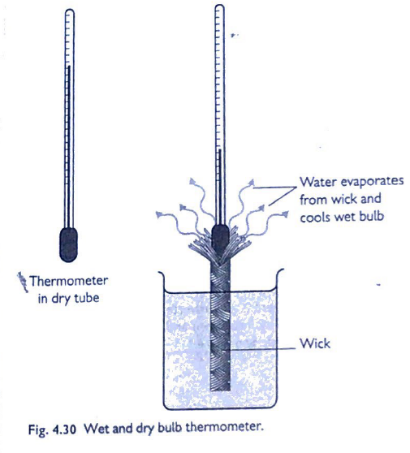
- 2 standard mercury thermometers
- One bulb exposed to air → Ambient T
- One bulb kept wet by a wick which is submerged in H2O
- H2O evaporates → bulb cools due to LATENT HEAT of VAPORISATION
- Wet Bulb temp < Dry Bulb temp
- ∆temp is related to the rate of evaporation of H2O which depends on humidity
- A scale is referenced to obtain R Humidity
Dew Point Hygrometer
Dew Point = the temp at which H2O vapour condenses to form liquid H2O
Dew Point occurs when Relative Humidity reaches 100% ∴H2O vapour begins to condense. When dew forms → a table is used to correspond the humidity to the ambient temp
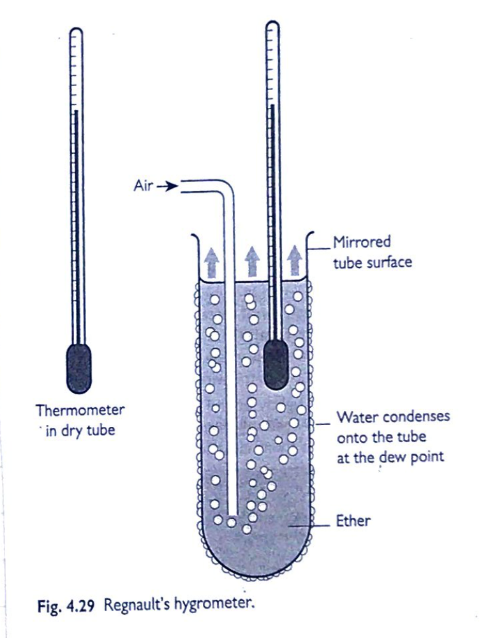
Renault’s Hygrometer
- A type of Dew Pt Hygrometer
- A mirrored tube (so you can see condensation)
- Filled with Ether (highly volatile)
- Air is blown through the ether, cooling it
- As ether evaporates → tube is cooled
- Temp at which dew forms on the outside of the tube noted as Dew Pt
- Table used to correspond that temp to humidity