23B06: Exam Report
Explain perfusion limited and diffusion limited transfer of gases in the alveolus.
48% of candidates passed this question.
This question required an explanation of the factors that influence perfusion and diffusion and a detailed description of the behaviour of specific gases including perfusion limited oxygen and carbon dioxide and diffusion limited carbon monoxide.
Factors influencing perfusion include flow/cardiac output, resistance (radius and length) and viscosity whereas diffusion is influenced by the characteristics of the gas (MW and solubility), surface area of diffusing surface and the pressure / concentration gradient.
Perfusion and/or diffusion limited characteristics of different gases included how quickly if at all equilibrium is reached and why this occurs with each specific gas.
21A15: Exam Report
Explain perfusion limited and diffusion limited transfer of gases in the alveolus.
36% of candidates passed this question.
This question required detail on those factors affecting gas exchange at the level of the alveolus. A description of the components of the Fick equation was expected – and how this related to oxygen and carbon dioxide transfer at the alveolar capillary membrane.
The rapid rate of equilibration (developed tension) was the limiting factor in of blood/alveolar exchange that rendered some gases perfusion limited (examples – N2O, O2 under usual conditions but not all) and the slower rate of others diffusion limited (examples CO and O2 under extreme conditions e.g., exercise, altitude). Estimates of time taken for each gas to equilibrate relative to the time taken for the RBC to travel across the interface was also expected for full marks. CO2 despite rapid equilibration and higher solubility was correctly described as perfusion limited (unless in disease states).
Better answers described CO2 as ventilation limited. Some answers also correctly included the component of interaction with the RBC and haemoglobin.
Ventilation/perfusion inequalities over the whole lung were not asked for and scored no marks.
F7iii / 23B06 / 21A15: Explain perfusion-limited and diffusion-limited transfer of gases
Gas Transfer in Alveoli
Gas is transferred between the alveoli and pulmonary capillary blood by diffusion
- Gas moves down a partial pressure gradient – in either direction
Factors that affect the rate of diffusion are outlined by Fick’s Law of Diffusion
- \(\text{Rate of diffusion = K ×} \normalsize \frac{\text{ Surface area × partial pressure difference }}{\text{Membrane thickness}} \)
- K = Krogh’s diffusion coefficient. This takes into account the mass of the molecules (\(\text{rate of diffusion ∝ } \normalsize \frac{\text{1}}{\sqrt{\text{molar mass}}} \)), temperature, solubility of the gas, viscosity of the fluid. K is different for each gas.
Factors affecting the perfusion of the alveoli/alveolar capillaries are
- Cardiac output (total pulmonary blood flow) – in turn influenced by:
- Vessel length – in turn influenced by capillary recruitment
- Vessel radius – in turn influenced by capillary distension
- Viscosity of blood
Other factors affecting the amount of gas transferred include
- Capillary transit time – normally ~0.75s, but can decrease with increased blood flow/cardiac output
- Gas-protein binding (e.g. O2 and Hb) – maintains a low partial pressure (only dissolved gas exerts a partial pressure)
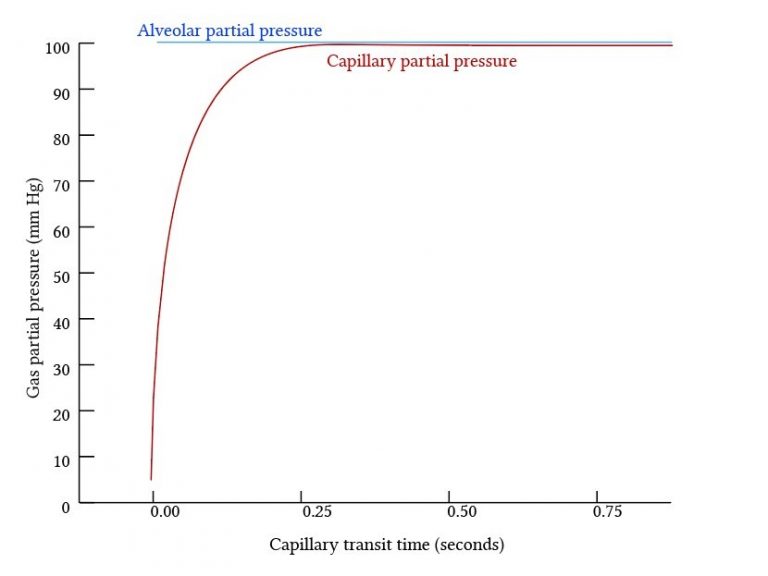
Gases that equilibrate rapidly between the alveoli and blood are perfusion limited, and gases that equilibrate slowly are diffusion limited.
Perfusion Limited Gas Exchange
- The total amount of gas exchange is dependent on the blood flow to the alveolus.
- Occurs when there is rapid diffusion between the alveoli and pulmonary capillary blood
- The partial presssure equalises early in the blood transit through the pulmonary capillary – diffusion stops. The only way to transfer more gas is by delivering more blood -> maintains the partial pressure gradient.
- Gas exchange is complete before blood reaches the end of the pulmonary capillary
Examples
- Oxygen
- Equilibrates rapidly – occurs in ~0.25s (1/3 of blood transit time)
- Hb increases the total amount of O2 exchange by maintaining a low PaO2 until most Hb saturated -> PaO2 then equilibrates rapidly
- CO2
- Equilibrates extremely rapidly – high solubility in tissue allows rapid movement from blood through alveolar-capillary membrane, small partial pressure difference allows rapid equilibration
- CO2 transfer limited is more limited by ventilation – this controls the partial pressure of CO2 in the alveoli and therefore the gradient for gas transfer across the alveolar-capillary membrane
- N2O
- No binding to Hb – partial pressure gradient equilibrates quickly
- Relatively low solubility in blood – partial pressure in blood increases more rapidly for the same volume of gas
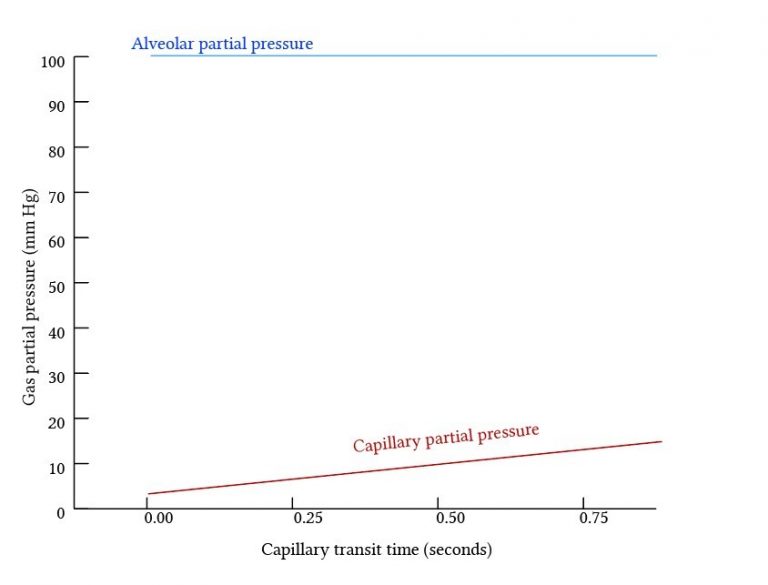
Diffusion Limited Gas Exchange
- The total amount of gas exchange is dependent on the rate of diffusion of the gas.
- Occurs when diffusion is slow – Partial pressures do not equilibrate along the pulmonary capillary and diffusion can continue along the whole length of the capillary
- By definition, equilibration time > capillary transit time (0.75s)
Examples
- Carbon Monoxide
- Extremely avid Hb binding maintains a very low partial pressure in blood for the entire capillary length – diffusion continues along the entire capillary length without equilibration of partial pressures.
- Slow diffusion
- The total amount of CO taken up is therefore dependent on characteristics of the diffusion membrane and the gas
- Oxygen under extreme conditions
- When capillary transit time decreases – oxygen transfer may not complete before the end of the capillary (partial pressure gradient remains). Occurs in very high cardiac output (e.g. intense exercise, when capillary transit time is < 0.25s)
- Disease states – Increased alveolar membrane thickness (e.g. pulmonary oedema), diffusion slows to the point that equilibrium is not reached
- High altitude – the O2 partial pressure gradient is low, diffusion slows to the point that equilibrium is not reached
Images from Deranged Physiology
Author: Joshua McLarty