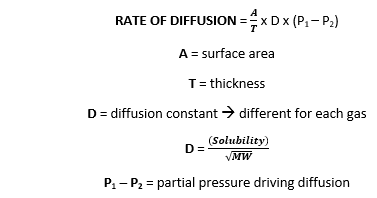
F7iii: Explain perfusion limited & diffusion limited transfer of gases
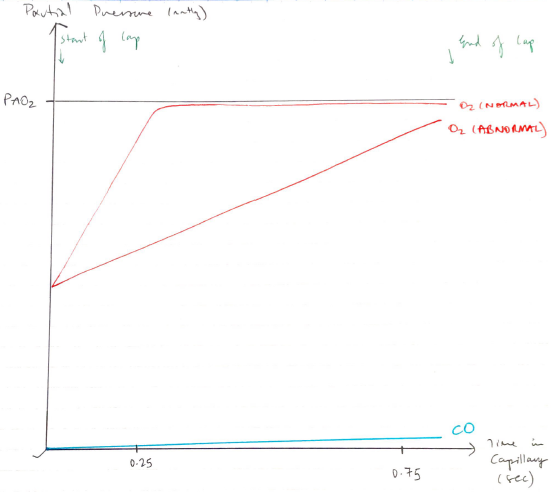
Perfusion Limitation
- RBC transit time in pulmonary caps is 0.75secs
- O2 combines with Hb
- ↑partial P of O2
- ∴O2 diffusion is PERFUSION LIMITED
- It requires more blood to maintain the partial pressure gradient
- Normally O2 ↑partial pressure by 0.25secs
Diffusion Limitation
- If alveolar membrane is thickened e. fibrosis
- PaO2 does not equilibrate with PAO2
- ∴Becomes DIFFUSION LIMITED
- CO has greater (x250) affinity for Hb cf. O2
- ∴binds so avidly its partial pressure almost never rises
- Since partial pressures never equilibrate, it is a DIFFUSION LIMITED gas
NOTE: partial pressure of O2 is never 0