14B18: Exam Report
Explain the similarities and differences between myoglobin and adult haemoglobin (60% of marks) and their physiologic relevance (40% of marks).
23% of candidates passed this question.
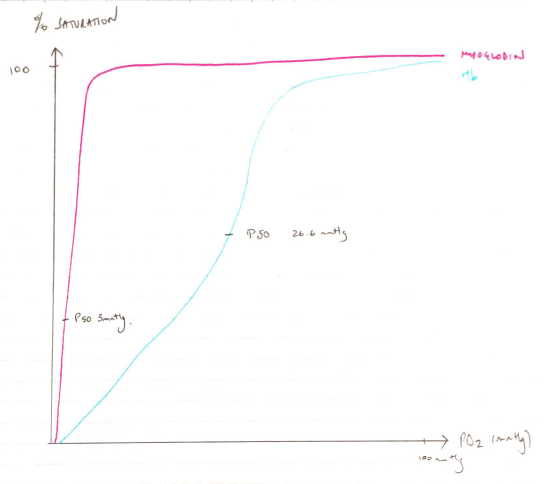
Both are globular proteins that bind and deliver O2. Due to myoglobin containing a single globin chain its dissociation curve is hyperbolic in shape. Haemoglobin contains 4 globin chains and is a quaternary structure which exhibits cooperatively resulting in a sigmoid shaped dissociation curve. The differing dissociation curves mean that when the PO2 is high, as in the lungs, both myoglobin and haemoglobin are saturated with oxygen. However, at the lower levels of PO2 in the tissues, haemoglobin cannot bind oxygen as well as myoglobin. Myoglobin can bind the O2 released by haemoglobin, which it stores to meet the demands of muscle contraction. This means haemoglobin (with its higher p50) can offload O2 to myoglobin. Comments on the synthesis and degradation gained additional marks but were a common omission.
The physiological relevance was poorly explained. The similarities and differences mean that haemoglobin is the primary means of O2 transport from the lungs to the tissues and myoglobin is the primary O2 carrying pigment of skeletal muscle and acts as local O2 reserve for times of intense muscle activity.
F8i / 14B18: Explain the similarities & differences between myoglobin & adult haemoglobin & their physiological relevance
Function
Myoglobin
O2 store for active muscle
Haemoglobin
O2 carriage from lung → tissue
CO2 carriage
Acid-base buffer
Location
Myoglobin
Skeletal & cardiac m
Only in blood in pathological states (muscle injury)
Large amounts of myoglobin cause renal failure (rhabdo → ARF)
Haemoglobin
Blood
200 – 300 million Hb molecules per RBC
Structure
Myoglobin
Haemoglobin
Both globular proteins with heme moiety that binds O2
Myoglobin
17,000 Da
Single globular chain ∴1 heme moiety
↓
∴binds 1 x O2
NO +ve cooperativity
Haemoglobin
65,000 Da
4 polypeptide chains ∴4 heme moieties
↓
∴binds up to 4 x O2 WITH +ve cooperativity
O2 Carriage
Myoglobin
Hyperbolic ODC
Higher affinity for O2 at low PO2
Operating range 1 – 5mmHg
P50 2.75mmHg
↓
Well match to intracellular PO2 1 – 5mmHg
Myoglobin is highly concentrated in muscles which contract +++
Blood supply during contraction is often cut off ∴myoglobin provides ongoing O2
High O2 affinity can load O2 from Hb
NOT pH sensitive
∴NO BOHR EFFECT
Haemoglobin
Sigmoid ODC
Low O2 affinity
Operating range 20 – 100mmHg
P50 26.6mmHg
pH sensitive
∴BOHR EFFECT → offloads O2 at ↑H+/↑CO2