20A01: Exam Report
Describe the carriage of carbon dioxide in blood
68% of candidates passed this question.
A detailed understanding of the carriage of carbon dioxide (CO2) in the blood is essential to the practice of intensive care medicine.
Comprehensive answers classified and quantified the mechanisms of CO2 carriage in the blood and highlighted the differences between the arterial and venous systems.
An explanation of the physiological principles surrounding these differences and the factors which may affect them was expected.
The changes that occur at the alveolar and peripheral tissue interfaces with a similar explanation of process was also required.
Candidate answers were often at the depth of knowledge required for an ‘outline question’ and a more detailed explanation was required to score well.
18B05: Exam Report
Describe the carriage of carbon dioxide (CO2) in the blood.
65% of candidates passed this question.
A definition of arterial and venous CO2 content (mls and partial pressure) and an outline of the 3 forms of CO2 in the blood and their contribution to the AV difference, followed by a detailed explanation of each form of carriage was required for this question. A good answer included a table of the contribution of each form of carriage to arterial and venous content and the AV difference; explained the concepts of chloride shift when describing carriage as HCO3-; detailed the Haldane effect and its contribution to carbamino carriage and referenced Henry’s law when describing dissolved CO2.
West’s Chapter 6 on gas transport details the key information to score well on this question.
15A13: Exam Report
Describe how carbon dioxide (CO2) is carried in the blood.
79% of candidates passed this question.
It was expected answers would describe each of the main categories of how CO2 is carried: Dissolved (10%), Plasma Bicarbonate (70%) and conjunction with plasma proteins and Hb as Carbamino Hb (20%). An opening statement quantifying the amount of CO2 dissolved in arterial (48mL/100mL) and venous blood (52mL/100mL) (4mL/100mL) and how this compares with Oxygen was expected (20 times more soluble).
For dissolved CO2, the application and description of Henry’s Law was awarded marks. A description of the consequences of the Haldane effect: difference in CO2 carriage of oxygenated and deoxygenated blood was expected. A diagram of pCO2 v CO2 content was helpful
F8iii 20A01 / 18B05 / 15A13: Describe how CO2 is carried in the blood
Definition
CO2 is a colourless, odourless gas composed of one carbon and two oxygen atoms present in the atmosphere and formed during respiration
CO2 production in the body is a result of aerobic metabolism in mitochondria. Normally 200mL/min
Carriage in Blood
Arterial
Mixed Venous
V-A Difference
Carbamino
Arterial
0.0017mmol/L
Mixed Venous
0.002 mmol/L
V-A Difference
30%
Dissolved
Arterial
1.2 mmol/L
Mixed Venous
1.4 mmol/L
V-A Difference
10%
HCO3–
Arterial
24.4 mmol/L
Mixed Venous
26.2 mmol/L
V-A Difference
60%
PCO2
Arterial
40mmHg
Mixed Venous
45mmHg
V-A Difference
mL/dL
Arterial
48mL/dL
Mixed Venous
52mL/dL
V-A Difference
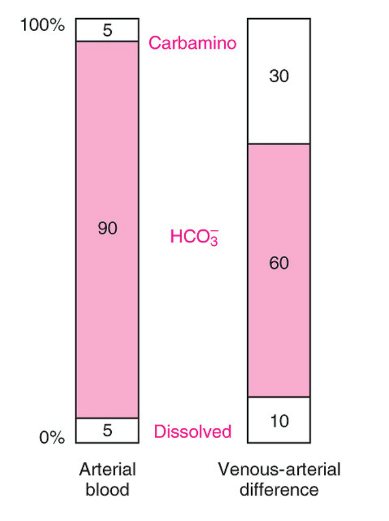
Straight Outta West’s:
The first column shows the proportions of the total CO2 concentration in arterial blood. The second column shows the proportions that make up the venous-arterial difference
Carbamino Compounds
- Carbamino compound = CO2 + terminal amine group of a protein (Hb)
- Hb is the most abundant protein (15g/dL)
- ∴Its terminal amine group is the most important for CARBAMINO formation
- Hb is a tetramer → ∴4 terminal amine groups
- Carbamino compounds form when CO2 binds with terminal amine of the α-chain
- DeoxyHb forms carbamino compounds 3.5 x more readily than OxyHb
- 70% of HALDANE EFFECT: for any given PCO2, deoxygenated blood is able to carry more CO2
- Due to conformational ∆
- Unbinding of O2 causes β-chain conformational ∆
- Affinity for CO2 ↑
- DeoxyHb 3.5 x better at forming carbamino compounds than OxyHb
Dissolved CO2
- Obeys HENRY’S LAW: the [ ] of a gas in liquid is ∝ to its partial pressure
- CO2 is x 20 more soluble than O2
- 1mmHg CO2 = 0.06mL/100mL of blood
- 1mmHg O2 = 0.003mL/100mL of blood
- ∴Solubility Coefficient = 0.03mmol/L/mmHg
- ∴Dissolved CO2 (mL) = 0.03 x pCO2
HCO3 –
- Occurs in RBC
- HCO3– diffuses out, H+ cannot
- Cl– is exchanged for HCO3– to maintain electroneutrality & continue moving the equation forward KA ‘CHLORIDE SHIFT’
- Deoxygenated Hb is less acidic than OxyHb
- Accepts a H+ & also drives equation forward
- 30% of HALDALE EFFECT: for any given PCO2, deoxygenated blood is able to carry more CO2
CO2 Dissociation Curve
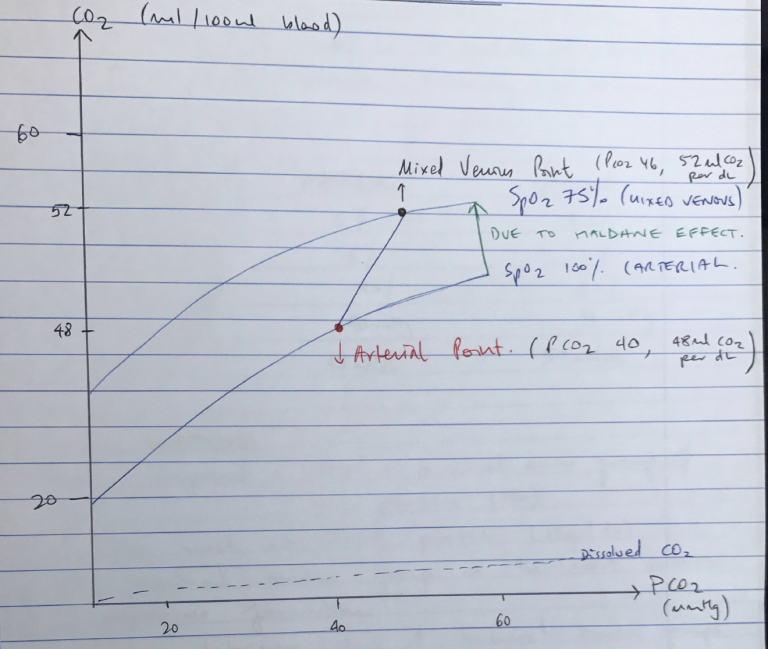
- CO2 dissociation curve shows relationship between blood PCO2 and CO2 content per dL
- Linear ODC → lacks +ve COOPERATIVITY
- Steeper than ODC → due to Haldane Effect, when there is ↑CO2 the blood can carry more
- Haldane Effect is responsible for difference between Arterial Pt & Venous Pt
- Arterial Point = PCO2 40mmHg, 48mL CO2/dL
- Venous Point = PCO2 46mmHg, 52mL CO2/dL