F8ii: Physiology of Haemoglobin
Definition
- A molecule of 4 protein chains, each carrying a heme group: MV 65,000 Daltons GG
- Normal levels \(
\begin{cases}
\text{M 13 – 18g/dL}\\
\text{F 11 – 16g/dL}
\end{cases}
\) - Each RBC consists of 200 – 300 million molecules of Hb
Synthesis
- 2 parts: heme + globin
- Each heme moiety consists of PROTOPORPHYRIN RING & central iron ion in ferrous state (Fe2+)
- Heme synthesis occurs in RBC cytosol & mitochondria
- Fe2+ forms 6 bonds with heme moiety; 5 bind Fe2+ firmly, 1 binds O2
- 4 polypeptide chains determine type of Hb
- HbA = 2α + 2β globin chains
- HbF = 2α + 2γ globin chains
- HbA2 = 2α + 2δ globin chains
- Altered polypeptide chains create variants important to know because alter body’s capacity to transport O2
NB: 98% of normal adult Hb is HbA
Function
- Transport O2 from lungs → tissues
- Transport CO2 from tissues → lungs as CARBAMINO Hb
- Buffer H+ formed in RBC
- No metabolism
O2 Transport
- O2 binds reversibly to heme
- 4 hemes → each Hb can carry 4 x O2
- Hb is an allosteric protein
- The binding of O2 to one heme group ↑affinity of remaining heme groups for O2
- This +ve cooperativity means that DeoxyHb & oxyHb have very different structures
- This shape is maintained by electrostatic bonds b/w α-acid sequences
- DeoxyHb: electrostatic bonds are strong, hiding heme deep in crevice, making it difficult for O2 to access heme this quaternary structure → KA TENSE FORM
- OxyHb = binding of first O2 to a heme changes the electrostatic bonds. Relaxes the crevice holding heme and enlarges the access → transmits this to other globin chains by distracting their electrostatic bonds & the quaternary structure is altered
- ↑ other globin affinity for O2
- KA RELAXED FORM when 4O2 bound to 1Hb
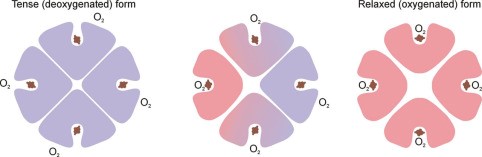
- The conformational state of Hb (R/T) is also altered by other factors, which influence strength of electrostatic bond:
- CO2
- pH
- Temperature
- Huffner Constant
- O2 binding capacity of Hb = amount of O2 in mL each Hb can carry
- KA HUFFNER’S CONSTANT = 1.3mL/g of Hb
- The Oxyhaemoglobin Dissociation Curve
- O2 binds reversible with ferrous iron of Hb to form oxyhaemoglobin
Hb + O2 ⮂ HbO2
- Forward reaction occurs in lungs due to ↑PO2
- Backward reaction occurs in tissues due to ↓PO2
- Curve is SIGMOID SHAPE due to COOPERATIVITY
- Affinity for O2 is lowest when first molecule binds to ‘TENSE’ deoxygenated Hb
- As each subsequent molecule binds, the gradient of the curve increases
- As PO2 increases, all the Hb – O2 binding sites become occupied & the curve levels off
- 3 important points on ODC:
- Arterial PO2 where Hb 100% saturated (PO2 100 = SpO2 97%)
- Venous saturation (PO2 40 = SpO2 75%)
- P50 (P50 = 27mmHg PO2 = SpO2 50%)
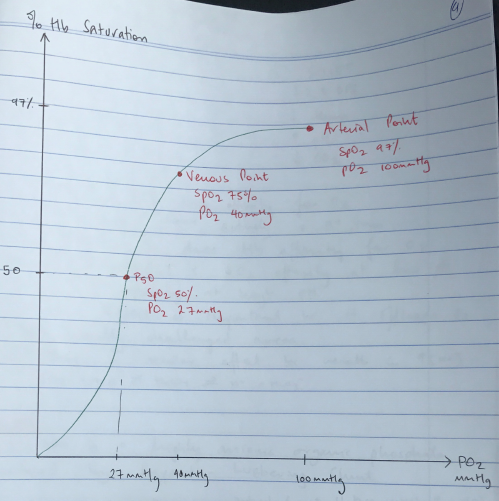
- P50 = the partial pressure of oxygen in blood where Hb is 50% saturated
- P50 is a measure of oxygen affinity
- ∴It is useful to compare changes in the position of the ODC
- P50 for any single form of Hb is variable → the hydrogen bonds & ionic interactions within Hb result in altered affinity of Hb for O2
R) Shift
↑2,3 DPG
↑Temp
↓pH
↑CO2
L) Shift
↓2,3 DPG
↓Temp
↑pH
↓CO2
pH
- An ↑[H+] will ↓Hb affinity for O2
- DeoxyHb binds more actively with H+ ions
- ∴as pH ↓so does Hb affinity for O2
KA BOHR EFFECT: ↓ O1 affinity at low pH & ↑ O2 affinity at high pH
- The Bohr Effect is important because it offloads O2 to metabolically challenged areas
- CO2 has a similar effect because results in ↑[H+]
CO2 + H2O ⮂ H2CO3 ⮂ H + + HCO3–
2, 3 DPG
- 2,3 DPG is a highly anionic organic phosphate
- Produced by Rapport – Luebering Shunt
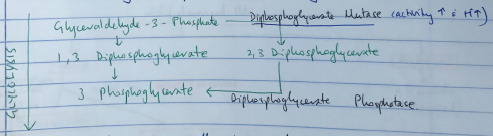
- This shunt comes off the glycolysis pathway
- Level of 2,3 DPG determined by balance of synthesis/degradation
- Activity of DPG Mutase ↑ with ↑H+
- DPG binds b/w two β globin chains & stabilises the Hb in the TENSE configuration
- ∴↓Hb affinity for oxygen & displacing ODC to RIGHT
- 2,3 DPG is ↑in ANAEMIA & HIGH ALTITUDE
- ∴tissue hypoxia of anaemia partially corrected by ↑P50
- The ↑RESP ALKALOSIS at altitude has much more pronounced effect than the ↑2,3 DPG & at high altitude ODC shifts LEFT
- Blood storage alters 2,3 DPG levels
- 26°C glycolysis ↓<5% normal rates
- ∴so does the production of 2,3 DPG
- After 2 weeks, 2,3 DPG levels are zero
- Once transfused, RBC become warmed & provided with metabolites for glycolysis
- The limiting factor to restore 2,3 DPG levels is the activity of 2,3 DPG MUTASE
- DPG levels of transfused blood = 50% normal in 7hrs
- Normal levels are reached in 48hrs
Hb Destruction
In ILEUM/COLON converted to UROBILINOGEN by bacterial by removing Glucuronic Acid
→ UROBILINOGEN
- Lipid soluble
- 10% reabsorbed & transported by albumin → enterohepatic circulation → URINE
- 90% further oxidised to STEROCOBILIN
- Excreted in faeces