R1i: Define Heat & Temperature
Definition
Heat
A form of energy determined by how active the molecules of a substance are
Temperature
Property of a system
A measure of the average kinetic energy of individual atoms that make up a substance
SI Unit
Joules
Kelvin
Particles
Heat = how many atoms are in a substance x how much energy each atom possesses
Temperature = how fast on average the particles are moving
Ability to do work
Can do work
Does not do work
Can only be used to measure the degree of heat and therefore determines which direction heat will travel
Heat and temperature are inter-related → icebergs have low temperature but contain a lot of heat bc of the large number of molecules present
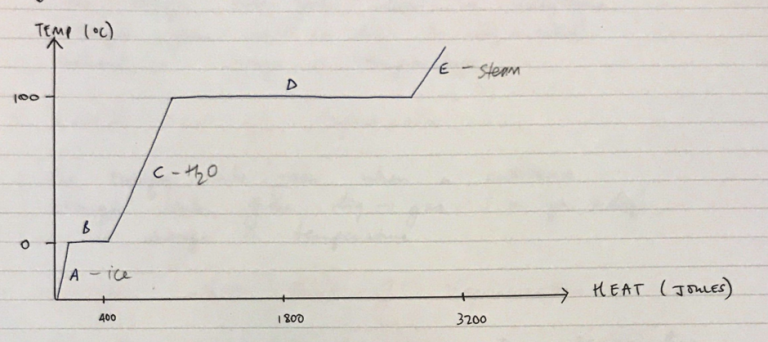
Relationship between heat and temperature is described as a substance change in state from: solid → liquid → gas
A = Ice
If you add heat the temperature increases
B = temperature remains the same because all the energy going into the system is being used to break the crystalline bonds of ice
C = H2O
Once all crystalline bonds are broken
Adding more energy (heat) to this system sees the temperature rise further until 100°C
D = temperature plateau at 100°C
All energy added to system is being used to break bonds between H2O molecules to form gas
Note how much more energy is required to convert liquid → gas cf. ice → liquid
Latent Heat of Crystallisation = the energy taken/given when a substance changes from solid → liquid (or vice versa) without a change in temperature
Latent Heat of Vapourisation = the energy taken/given when a substance changes state from liquid → gas (or vice versa) with no change in temperature
Specific Latent Heat of Vapourisation = the energy required to change the temperature of a unit of mass (usually 1kg) by 1°C at a specified temperature.