Wiv: Measurement of Pressure of Gases
Definition
PRESSURE = FORCE / UNIT AREA
SI Units
Pascals
(kPa used in physiology bc Pa is too small)
Interconversion
1 ATM = 101.3kPa = 1 Bar
Methods of Measurement
Note: Aneroid – ‘without νερο (water)’ ie does not contain liquid for measuring
Use
High P Aneroid Gauge
High P in gas cylinders, pipelines
Low P Aneroid Gauge
Low P ie airway pressure
Barometer
Atmospheric pressure
Manometer
NIBP
Mechanism
High P Aneroid Gauge
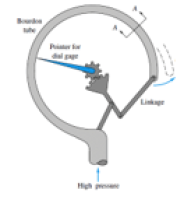
ie Bourdon gauge
A flexible coiled tube is connected to a pointer via gears
The other end is exposed to gas supply pressure
High pressure causes the tube to uncoil (think party whistle), rotating the pointer on a dial where you read the gas pressure
Low P Aneroid Gauge
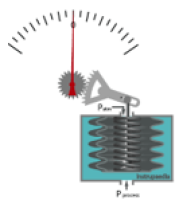
ie Bellows Gauge
An elastic chamber through which pressure is applied
Bellows expand as their P increases, this moves the pointer across a dial
Barometer

Liquid containing (but can be aneroid)
U shaped glass tube with liquid mercury
One end is closed and there is a vacuum space between the closed door and the mercury
Other end is open to atmosphere
Atmospheric P is exerted, forcing mercury to rise,
Manometer
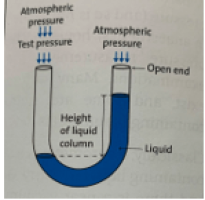
U shaped glass tube
Filled with mercury/H20
Open both sides, so atmospheric P is exerted on both ends (cancelling itself out)
Pressure to be measured is applied to one end, causes liquid to rise on the opposite side
The height difference in cm is the pressure in cmH20 or mmHg
Adv
High P Aneroid Gauge
Accurate 0.1-2%
Sensitive to small P changes
No power supply necessary
Low P Aneroid Gauge
Fast to allow breath-by-breath measurement
Barometer
Simple to set up and use
Manometer
Simple to set up and use
Disadv
High P Aneroid Gauge
Sensitive to shock/vibration
Hystersis
Sensitive to temp change
Low P Aneroid Gauge
Temp sensitive
Barometer
Toxic mercury
Manometer
Bulky
Accuracy is operator dependant