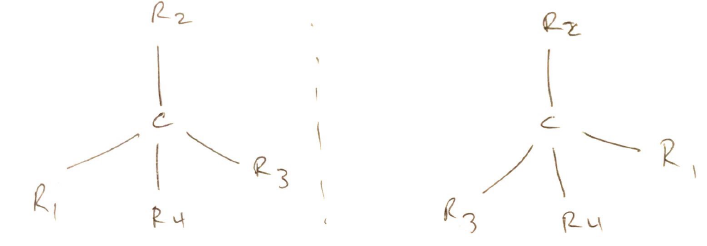Dviii: Describe & give examples of the clinical importance of isomerism
Definitions
- Isomer = identical formula, different bonding arrangements of atoms, or their orientation in space
- Structural isomer = same chemical formula, structure is different
- Stereoisomer = same chemical formula, same atom arrangement, arrangement in space is different (i.e. different 3D configuration)
- Enantiomer = molecules that are mirror images & cannot be superimposed
Isomer
Structural
Steroisomer
Structural
Chain
Position
Tautomerism
Steroisomer
Optical
Geometric
Cis
Trans
Stereoisomerism
- Same chemical formula
- Atom arrangement same, but arrangement in space different (i.e. diff 3D config)
Cistatracurium = cis-cis isomer of atracurium
- 50% more potent
- ↓histamine release
Optical (Enantiomer)
- Molecules are mirror images therefore cannot be superimposed
- Contain > 1 chiral centre
- CHIRAL CENTRE = central atom surrounded 4 different chemical groups so in 3D one extends vertical & others point out
- Classified by configuration of functional groups attached to chiral centre
- R (RECTUS) = clockwise (Latin – Right handed)
- S (SINISTER) = anticlockwise (Latin – Left handed)
- Enantiomer = 1 chiral centre
- Diastereoisomer = >1 chiral centres
- Mainly exist as Racemix (2 isomers 50:50) e. ketamine → S-ketamine = more potent dissociative activity)
Clinical Significance
Ceutics
- Pure enantiomer is very expensive ∴ most drug are racemic
- Producing a pure enantiomer allows re-patenting of a drug
PK
- You can ↓dose
- Passive absorption = same
- Active transport may favour one isomer over another
- 1st pass metabolism may favour one e. L-verapamil has much more OBA cf. s-verapamil
- PPB → differs ∴ different clearance
PD
- Enantiomer – receptor interaction i.e. different active/inactive/partially active/antagonist
- Enantiomer/enantiomic interactions = similar config ∴ compete for same PPB site