J1iv: H-H & Stewart Approach to Acid Base
Henderson-Hasslebank Approach
- LAW OF MASS ACTION → the velocity of a chemical reaction is proportional to the active concentrations of the reaction
- The H-H equation describes the relationship between:
- pH
- Dissociation constant
- Weak acid & its base salt
- In solution, an acid dissociates:
- The proportions of acid & base depend on the dissociation constants
- If K1 > K2 then there will be more H+ & A– in solution
- pKa = the negative log of the dissociation constant & is equal to the pH at which the substance is 50% dissociated
pKa = – log [KA] ← acid dissociation constant
NB: pH is temp related → ↓temp = ↓ionic dissociation H2O = ↑pH
Stewart Approach
Introduction
- AKA Physiochemical Approach
- Differs from HH which is determined by H+ & HCO3–
- 3 independent variables which control acidity
Independent Variables → these ∆ pH (= 1° cause)
- pCO2
- ATOT (total weak non-volatile acids)
- SID
Dependent Variables (= 2° effect)
- These values depend on values of independent variables
- If these ∆, then independent variables must have ∆
- H+
- OH–
- CO32-
- HA (weak acid)
- A– (weak anions)
Physical Laws Of Physiochemical Methods
- Interaction of dependent & independent variables must obey the laws of aqueous solution:
- ELECTRONEUTRALITY – in any aqueous solution the sum of all +vely charged ions must equal the sum of all -vely charged ions
- DISSOCIATION EQUILIBRIA – derived from Law of Mass Action. The velocity of a chemical reaction is proportional to the active [ ] of the reaction
- CONSERVATION OF MASS – the amount of substance remains constant unless added, removed, generated, or destroyed
SID in Plasma
- All solutions of body have H2O
- [H+] of these solutions depends on the dissociation of H2O
- H2O dissociation depends on the VARIABLES!
- SID, PCO2, ATOT
- Strong cations = Na, K, Ca, Mg
- Strong anions = Cl, SO42-
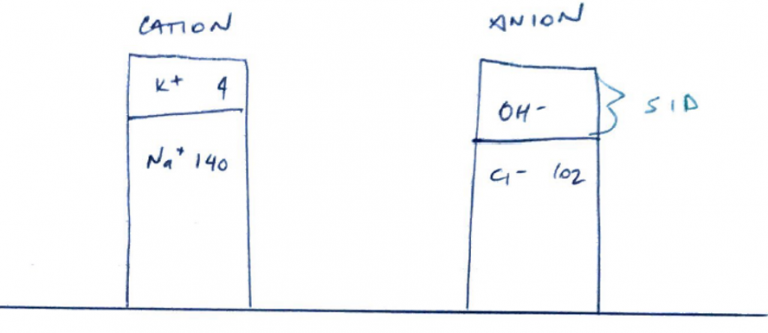
- SID = strong cations – strong anions
- Normal plasma SID = 42 mEq/L
- But you must have Electroneutrality
- Whereby Anions = cations so that the SID = 0
- But it’s not, it’s 42mEq/L and slightly alkaline
- ∴somewhere in plasma is unmeasured anions (the OH- in the above gamblegram)
- ↑SID = alkalosis (the unmeasured anions have increased further)
- ↓SID = acidosis (there are less unmeasured anions)
(see Gamblegram)
- ↑/↓ Na
- ↑/↓ Cl –
- ↑ organic acids with pKa < 4 (Strong acids)e. lactate, ketones
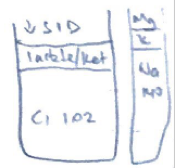
- ATOT
- Non-volatile weak acids (non-CO2) exist in all body fluid compartments
- In plasma: PO42-, serum proteins, albumin
- Controlled by metabolic state & liver
- Non-volatile weak acids dissociate:
HA ↔ H+ + A–
- Total concentration of weak acid = ATOT
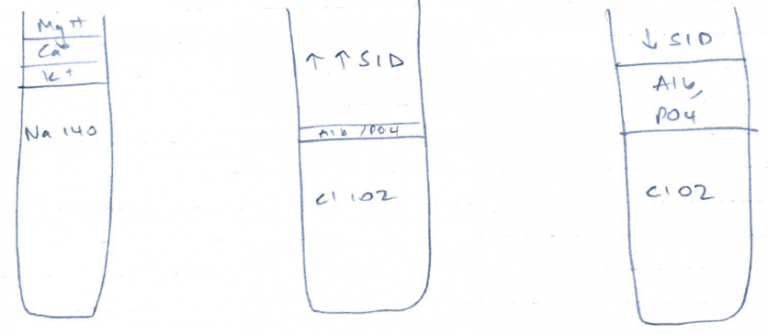
- ↑Alb/PO4 = ↓ SID = acidosis
- ↓Alb/PO4 = ↑ SID = alkalosis
- PCO2
- Controlled by respiratory system
- ↑CO2 = ↑H+ (adding)
Classification According to Stewart’s
Resp Causes
- ↑/↓ PCO2
Non-Resp Causes
- ABNORMAL SID ↑/↓ H2O
Water excess = ↓SID
Water deficit = ↑ SID
- STRONG ION IMBALANCE ↓/↑ SID
↑Cl = ↓SID
↓Cl = ↑SID
Anion excess = ↓SID
- ATOT ABNORMALITY ∆ ATOT
↓/↑ Phosphate
↑/↓ Albumin