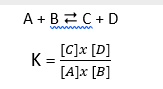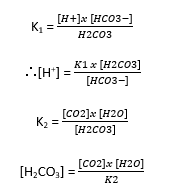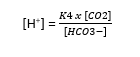J1i: Principles Underlying Acid-Base Chemistry
Importance to Regulate pH
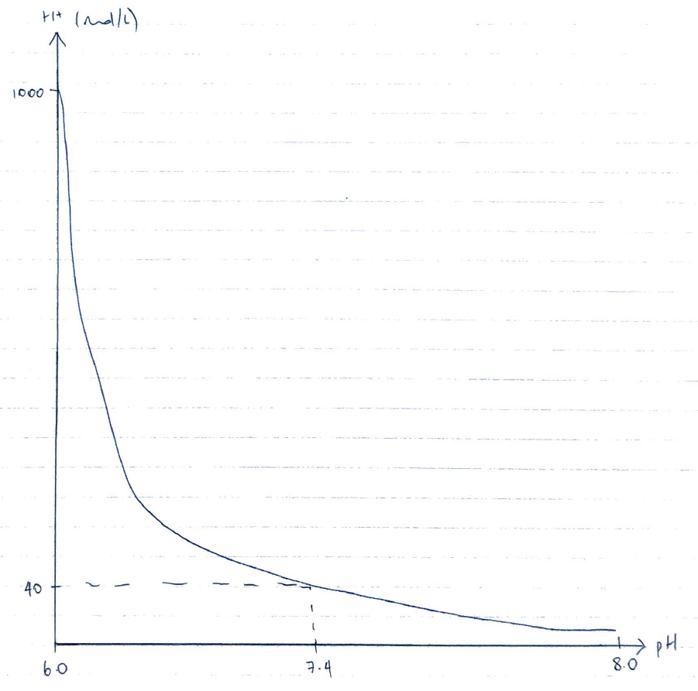
- pH is the negative logarithm to the base 10 of the hydrogen ion [H+]
pH = – log10 [H+]
- Normal arterial pH = 7.4 = [H+] 40 mol/L
- The negative logarithmic scale of pH is non-linear & the relationship between pH & [H+] is inverse
- ∴↓ pH 1 unit = x10 fold ↑ H+ activity
- ∴↓ pH 7.4 → 7.3 = ↑H+ from 40 → 50mol/L
- ∴tight regulation [H+] for 2 reasons:
- SMALL MOLECULES: completely ionise at neutral pH ∴become trapped in cells of organelles
- PROTEINS: perform optimally at a specific pH ∴altering pH effects
- Enzyme activity
- Membrane excitability
- Electron production
- Hormone release
- CNS reflexes
Bronsted-Lowry Definition
- Acid = proton donor
- Base = proton acceptor
- H+ = an atom without an electron i.e. a proton
- Strong acid = an acid with a strong tendency to completely dissociate & discharge its H+ into solution
- HA (strong acid) ↔ H+ (proton) + A–
- Strong base = base that reacts powerfully with H+, mopping it up from solution
→ Strong acids & bases always fully dissociate/associate ∴only exist in a charged form
- Weak acid = less readily release H+
- Weak base = less readily accepts H+
→ Most acids & bases are weak i.e. only partially dissociate
- H2CO3 → most important weak acid
- HCO3– → most important weak base
- Distinguish strength of acid/base → based on pKa
- pKA : the negative logarithm of dissociation constant of a substance & the pH where the substance is 50% dissociated
pKA
- pKa < 4 = strong acid
- pKa > 12 = strong base
- pKa 4 – 12 = weak acid / base
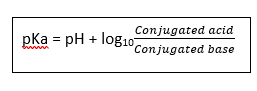
VOLATILE ACID = an acid that can only be excreted by lungs
FIXED ACID = an acid that requires excretion by kidneys i.e. cannot be excreted by lungs
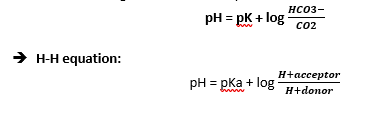
Henderson-Hasslebach Approach
- LAW OF MASS ACTION → the velocity of a chemical reaction is proportional to the active concentrations of the reactions
- The equilibrium constant (K) indicates which side of the reaction the equilibrium is:
- HENDERSON applied the LAW OF MASS ACTION to the equilibrium reaction for carbonic acid
CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3–
- If [H2O] is large enough to be considered as constant → ∴[H2CO3] = K3 x [CO2]
- Then substitute a function of the derivable [CO2] in place of [H2CO3] which can’t be measured:
- [CO2] can be calculated from PaCO2 using Henry’s Law
- K1, K2, K3, K4 are all numerically different constants
- Then SORENSEN introduced the pH scale
- It was known that ∆ [HCO3–] reflected accumulation of non-volatile acids
- pH measurement showed that ∆pCO2 was also affecting pH
- ∴concept of metabolic/resp acid-base derangement was formed
- HASSELBACH re-arranged Henderson’s equation: