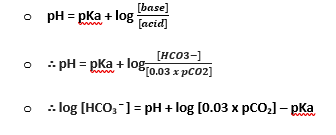14B22: Exam Report
Describe how the values for PaO2, PaCO2, pH and bicarbonate are determined on a blood gas sample.
23% of candidates passed this question.
A correct description of the Clarke electrode, Severinghaus and the pH electrode was expected to attain a pass. Candidates who used correct depictions of these electrodes with annotated description attracted higher marks. Most candidates didn’t comment on the temperature correction and standardization to 37 degrees. There was partial understanding on the calculation of HCO3 by most candidates. In general the question was poorly answered considering the wide spread use of blood gas analysis.
J2ii / 14B22: Describe how the values for PaO2, PaCO2, pH and HCO3- are determined on a blood gas sample
Blood Gas Analyser
- Uses a small amount of heparinised blood
- To pass through 4 electrodes sequentially
- Clarke electrode to measure O2
- Severinghaus electrode to measure CO2
- Glass electrode to measure pH
- 4th electrode is as reference electrode
- pCO2, pO2, pH → measured directly
- HCO3– → measured INDIRECTLY (as too is SaO2, O2 content, base excess)
- Blood gases reported as partial pressures
RECALL: Henry’s Law: the amount of gas dissolved in a solvent is directly proportional to its partial pressure @ STP
∴ blood gas samples are all warmed to 37°C for standardisation
PaO2 Clarke Electrode
- Silver/silver chloride anode
- Platinum cathode
- Anode & cathode suspended in KCl solution
- Power source provides potential difference of 0.6V
- A plastic semi-permeable (to O2) membrane surrounds PLATINUM cathode because exposure to blood would result in protein deposition, rendering → ineffective
- Electrons form at silver anode
- Ag → Ag+ + e–
- Oxygen diffuses to the platinum cathode, where e– from silver anode combined with O2 & H2O to give hydroxyl ions
- O2 + 4e– + 2H2O → 4 (OH – )
- The KCl of the solution
- Gives Cl – to silver ions → AgCl
- Picks up OH– → KOH
- ∴ completing circuit → allows current flow
- O2 IS THE RATE LIMITING STEP!
- ∴ size of current is proportional to pO2 in sample
pH Glass Electrode
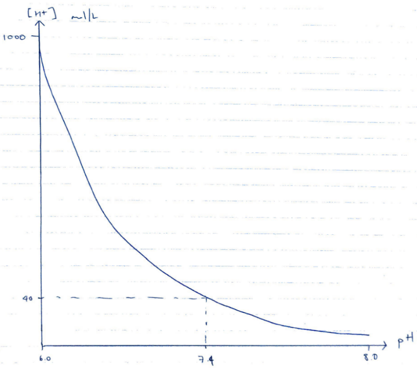
- pH is the measure of [H+] in a solution
- pH = – log10 [H+]
- Therefore pH 7.4 is [H+] of 40mmol/L at 37°C
- ∆ pH by 1 unit = x 10-fold ∆pH
- ∆ pH by 0.3 units = x 2-fold ∆pH
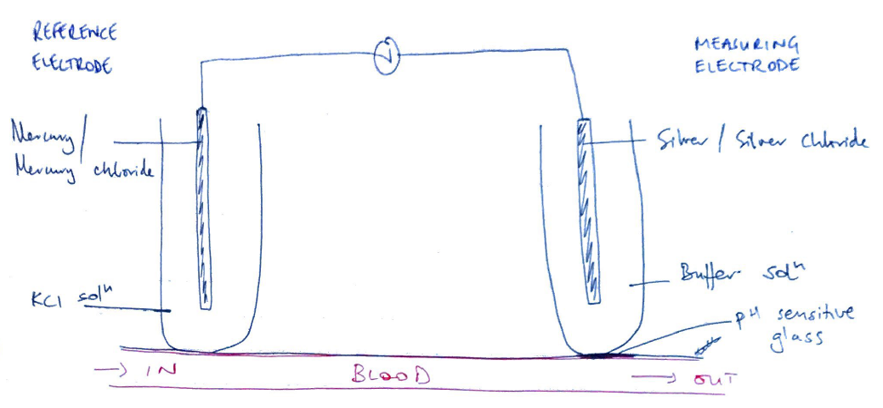
- 2 electrodes:
- Measuring electrode → silver/silver chloride, buffer solution
- Reference electrode → mercury/mercury chloride, KCl solution
- There is pH sensitive glass separating measuring electrode from blood sample
- The reference electrode maintains constant potential despite pH ∆
- At measuring electrode; H+ moves past pH sensitive glass
- But pH remains same because of buffer solution
- But allows a gradient to exist between blood & buffer solution
- Gradient produces an electrical difference
- 2 electrodes create a circuit
- Potential is measured & read as [H+]
Limitations of Glass Electrode
- Must be kept at constant temperature (hypothermia ↑CO2 solubility)
- Electrodes must be kept clean & protein free
- Calibration of buffer solutions is required
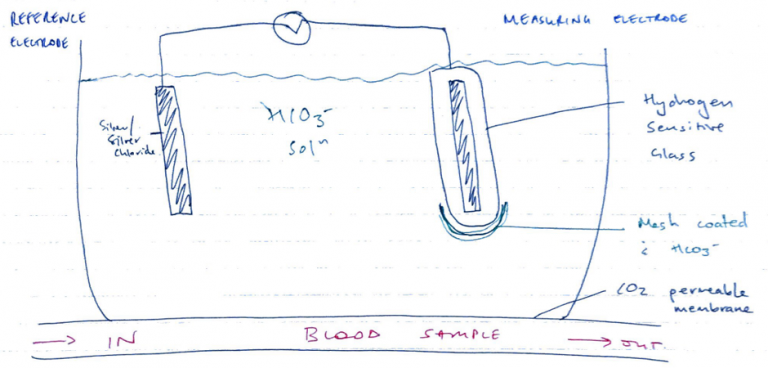
pCO2 Severinghaus Electrode
- Severinghaus is a modified pH electrode
- It is based on [H+] measurement because CO2 is related to [H+]
- CO2 + H2O ⇋ H2CO3 ⇋ H+ + HCO3–
- CO2 diffuses from blood sample into buffer solution
- Combines: CO2 + H2O ⇋ H2CO3 ⇋ H+ + HCO3–
- Glass electrode measures change in [H+], which depends on CO2 if temp & P kept constant
Limitations
- Takes 2 – 3 mins for CO2 to diffuse
- Must be calibrated with known concentration of CO2
- Must be kept constant temp & pressure
- Electrodes must be kept clean & protein free