F8ii / 15B05 : Explain the oxyhaemoglobin dissociation curve & the factors that may alter it
15B05: Exam Report
Explain the oxyhaemoglobin dissociation curve and the factors that may alter it.
77% of candidates passed this question.
Marks were awarded for an appropriate curve with values, an explanation of the nature of positive cooperatively and notes on those factors causing changes in the p50 or “shifts” in the curve.
Most candidates were able to provide the required sigmoid shaped curve with appropriate key value points (p50, venous and arterial points). Better candidates were able to identify p50 as a measure of avidity or affinity for oxygen and commented on T- Tense and R- Relaxed states, the role and production of 2,3 DPG , binding to the beta chains (nature of the lack effect on
foetal haemoglobin). Describing the mechanisms associated with factors shifting the curve and commenting on changes in oxygen content over the steep and flatter parts of the curves gained additional marks. Candidates are reminded to answer the question asked – no marks were awarded for a description of dissolved oxygen delivery. Some answers confused the Bohr and Haldane effects.
F8ii / 15B05: Explain the oxyhaemoglobin dissociation curve & the factors that may alter it
Definition
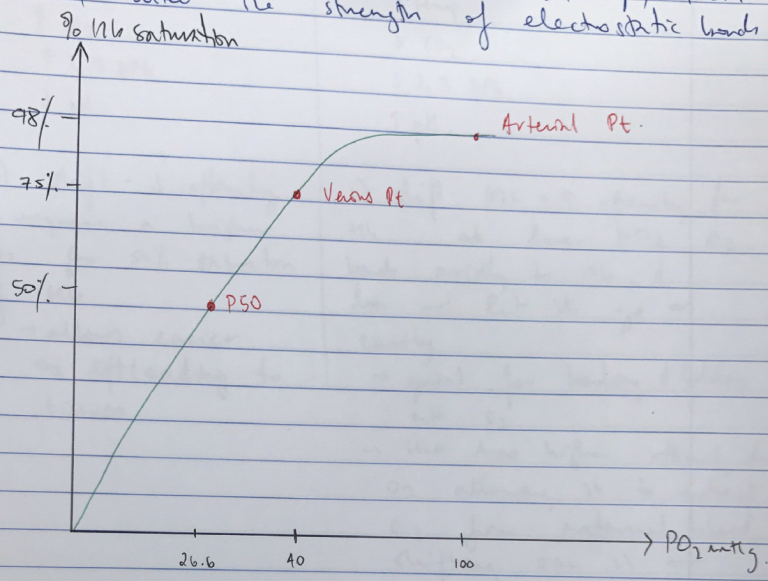
ODC: visual representation of the relationship between % Hb saturation and the PO2
O2 binds reversibly to heme
- 4 hemes in each Hb → ∴each Hb can carry 4 x O2
- Hb is an allosteric protein → binding of O2 to one heme group ↑affinity of remaining heme groups for O2
- This is KA POSITIVE COOPERATIVITY & is the reason for shape of ODC
- +VE COOPERATIVITY means OxyHb & DeoxyHb have very different structures
- DeoxyHb = electrostatic bonds are strong, hiding heme deep in crevice, making it difficult for O2 to access heme → KA TENSE FORM
- OxyHb = binding of first O2 to a heme changes the electrostatic bonds. Relaxes the crevice holding heme → O2 can access easier → transmits this to other globin chains by distracting their quaternary structure
- ↑affinity for O2
- KA RELAXED FORM
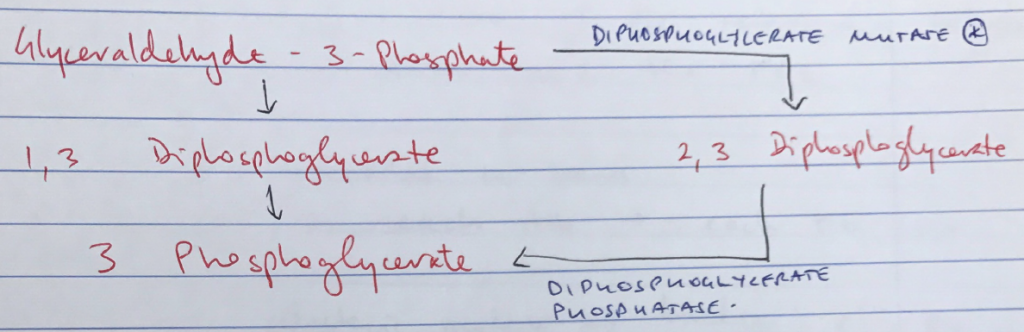
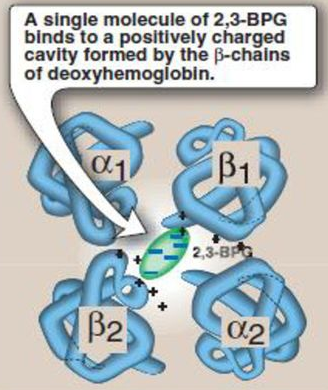
- Conformational state of Hb (R/T) is also altered by other factors: CO2, temp, 2,3 DPG, which alter the strength of electrostatic bonds
- P50 = partial pressure of O2 where Hb is 50% saturated with O2
- Important because used as marker of O2 affinity
- Different factors ↑/↓ affinity of Hb for O2 & you can compare them by using P50 as a reference point
- Venous Point: pO2 & % saturation Venous Blood: 75%, 40mmHg
- Arterial Point: pO2 & % saturation Arterial Blood: 98%, 100mmHg
R) Shift ODC
L) Shift ODC
↑Temp
↑CO2
↑2,3 DPG
↓pH
↓Temp
↓CO2
↓2,3 DPG
↑pH
R) shift = ↓affinity
∴requires a high PO2 for 50% saturation of Hb
→ allows easier O2 offloading to tissues
L) shift ODC = ↑affinity for Hb
∴at lower PO2 O2 binds avidly to Hb & does not let it go as easily
→ Great for binding & holding onto O2
→ HbF has higher affinity for O2, allowing it to extract O2 from maternal blood, shifting ODC L)
→ lacks β-chain (which is where 2,3 DPG binds)
- Author: Krisoula Zahariou