Q1iv / 22A15 / 19A10: Outline the sequence of haemostatic events after injury to a blood vessel wall
22A15: Exam Report
Describe the sequence of haemostatic events following injury to a blood vessel wall until clot stabilisation
46% of candidates passed this question.
A good answer was well structured and covered the areas of vasoconstriction, platelet adhesion, activation and aggregation, coagulation, clot retraction and anticlotting mechanisms.
Many answers gave an overview of the haemostatic process but revealed insufficient knowledge of the processes involved. It was acceptable to give a classical view of clotting or to describe the cell-based model; or both.
However, in several cases answers became confused by mixing up elements of the classical approach and cell-based model approach. Errors concerning details of the cell-based model were frequent.
Many candidates did not include how the clot is limited to just the site of injury which happens in parallel with the formation of clot. Candidates should be aware that writing lengthy introductory statements attracted no marks and wastes time.
19A10: Exam Report
Outline the sequence of haemostatic events after injury to a blood vessel wall (50% of marks). Discuss the role of naturally occurring anticoagulants in preventing clot formation in-vivo (50% of marks).
40% of candidates passed this question.
This question was best answered in a chronological manner. Many candidates omitted initial vasoconstriction and its mechanism. The platelet plug and formation of the clot should have then been described followed by the fate of the clot, including in-growth of fibroblasts. Strictly, fibrinolysis is a system for repairing / limiting clot propagation after the fact. Anticoagulants refer to antithrombin III, heparin, thrombomodulin and protein C and S. An explanation of the interaction of these naturally occurring anticoagulants was expected. The clotting factors that are specifically inhibited was expected as part of the discussion. The glycocalyx and vessel wall also plays a role in preventing coagulation.
Q1iv / 22A15 / 19A10: Outline the sequence of haemostatic events after injury to a blood vessel wall (50 marks). Discuss the role of naturally occurring anticoagulants in preventing clot formation in-vivo (50 marks).
Haemostasis
- Haemostasis = cessation of bleeding
3 Steps
- PRIMARY HAEMOSTASIS = platelet plug formation
- SECONDARY HAEMOSTASIS = coagulation to form FIBRIN MESH (which reinforces platelet plug)
- FIBRINOLYSIS & VESSEL REPAIR
Vascular Endothelium
- Coated with GLYCOCALYX & BM (= intima layer)
- Glycocalyx = complex gel of glycoproteins & proteoglycans that is produced by & coats endothelium
- Intact blood vessel provides non-thrombogenic surface that blood can pass through circulatory system
- Antithrombotic mediators produced by endothelium:
- Modulates fibrinolysis → Tissue-Plasminogen-Activator
- Subendothelial layer = highly thrombogenic
- Damaged endothelium causes:
- Collagen exposure
- TF expression on endothelial cells
- Release of endothelial mediators (PROTHROMBOTIC)
- TF → endothelial damage
- vWF → endothelial damage
- Plasminogen Activator Inhibitor – 1 → inhibits fibrinolysis
Primary Haemostasis
Vasoconstriction
- Endothelial drug → ↑symp → VC
→ Endothelin > NO & prostacyclin
→ VC
→ ↓BF through damaged vessel
Adhesion
- Endothelial drug
- Collagen exposed
- Platelets anchor to collagen via
- GP Ia/IIa (directly)
- GP Ib/IX (via vWF)
Activation
- Platelet granules released by:
- Collagen exposure
- Thrombin
- Granule secretion → ADP, 5HT, Ca2+
- ↑Intracellular Ca2+
- GP IIb/IIIa receptor activation
- ↑Thrombin
- ↑Thromboxane A2 → ↓cAMP → VC & further platelet aggregation
- Morphology platelet ∆ → allows ↑SA for further activation
Aggregation
- Activated IIb/IIIa receptors → bind vWF/Fibrinogen
- Allows intraplatelet signalling
- Allows aggregation
- Amplified by ↑ADP/TXA2, dense granule release
Secondary Haemostasis
- Coagulation aims to generate THROMBIN, which converts soluble fibrinogen → insoluble fibrin = stabilise platelet plug
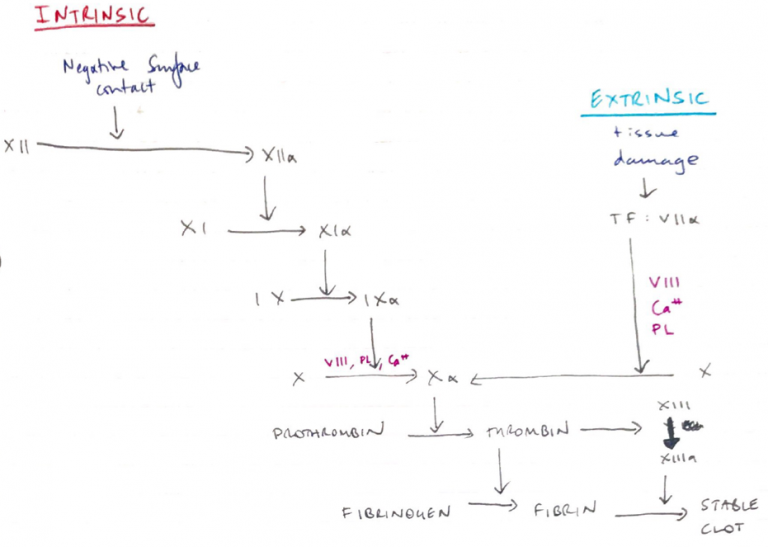
Extrinsic/Intrinsic Model
Intrinsic
- “Intrinsic” because does not require an additive
- Contacts negatively charged surface (collagen)
- Contact activation activates XII
- XIIa activates XI
- XIa on platelet surface + VIII + Ca2+ activates X
- Slower; 1 – 6 min
- APTT: exposes blood to negatively charged activator & Ca2+ to check ACTIVATED THROMBOPLASTIN TIME
Extrinsic
- “Extrinsic” because requires exposure to TF which is outside of blood
- TF normally on luminal side of endothelial cells
- Trauma → TF translocates to surface
- TF has high affinity for VII
- Binds & activates VII
- TF – VIIa on platelet surface with VIII, Ca2+ activates X
- Fast; 15 secs
- PT/INR: Prothrombin Time = Ca2+, TF and phospholipid are added to blood.
- PT is compared to an internationally recognised standard PT control sample & expressed as a ration ‘INR’
Common Pathway
- Activated Xa + Va2+/Ca2+/platelet surface converts PROTHROMBIN → THROMBIN
NB: Xa is the only known physiological activator of Prothrombin → thrombin
Thrombin Roles
- Converts fibrinogen → fibrin
- Activates V, VIII, XIII
- Stimulates platelet aggregation & secretion
- Promotes smooth muscle contraction
- Chemotaxis
Fibrinogen → fibrin
- Fibrin monomers by thrombin
- Monomers aggregate non-covalently
- XIIIa activated by thrombin
- XIIIa covalently cross-links Fibrin with ‘insoluble fibrin’
- Clot contraction → fills defect in vessel wall
- Fibrin & Thrombin promote active growth of fibroblasts
Regulation
AT III
- Glycoprotein of the liver
- t 1/2 3 days is plasma
- Inactivates enzymes of coagulation cascade
- EXTR: VIIa, IIx, major inhibition
- INTR: Xa, IXa, Xia, XIIa
Tissue factor pathway inhibitor
- Reversibly inhibits Xa
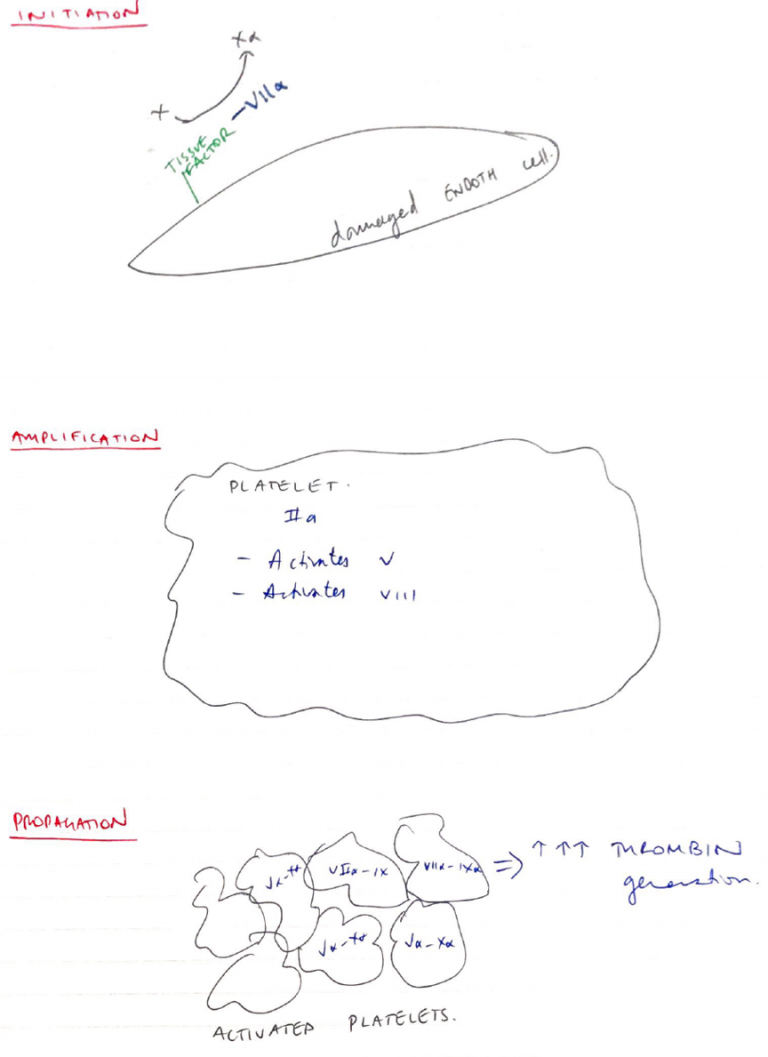
Cell Based Model of Coagulation
- Supersedes intrinsic/extrinsic model
- Focuses on cell surfaces as having a central role to haemostasis
- Consists of 3 phases:
1. Initiation Phase
- On any cell expressing tissue factor (i.e. Endothelial)
- Exposed TF activates VII
- VIIa activates X
- Xa generates a small amount of PROTHROMBIN -> THROMBIN
2. Amplification
- Thrombin generated insufficient to form a fibrin clot
- But serves as a +ve feedback
- Mostly on the platelet surface
- Activates V & VIII
- Va & VIIIa accelerate & amplify more thrombin generation
3. Propagation
- On platelet surface
- Thrombin generation converts FIBRINOGEN → FIBRIN
- Thrombin promotes active growth of fibroblasts
- Thrombin activates XIII
- XIIIa further stabilises clot
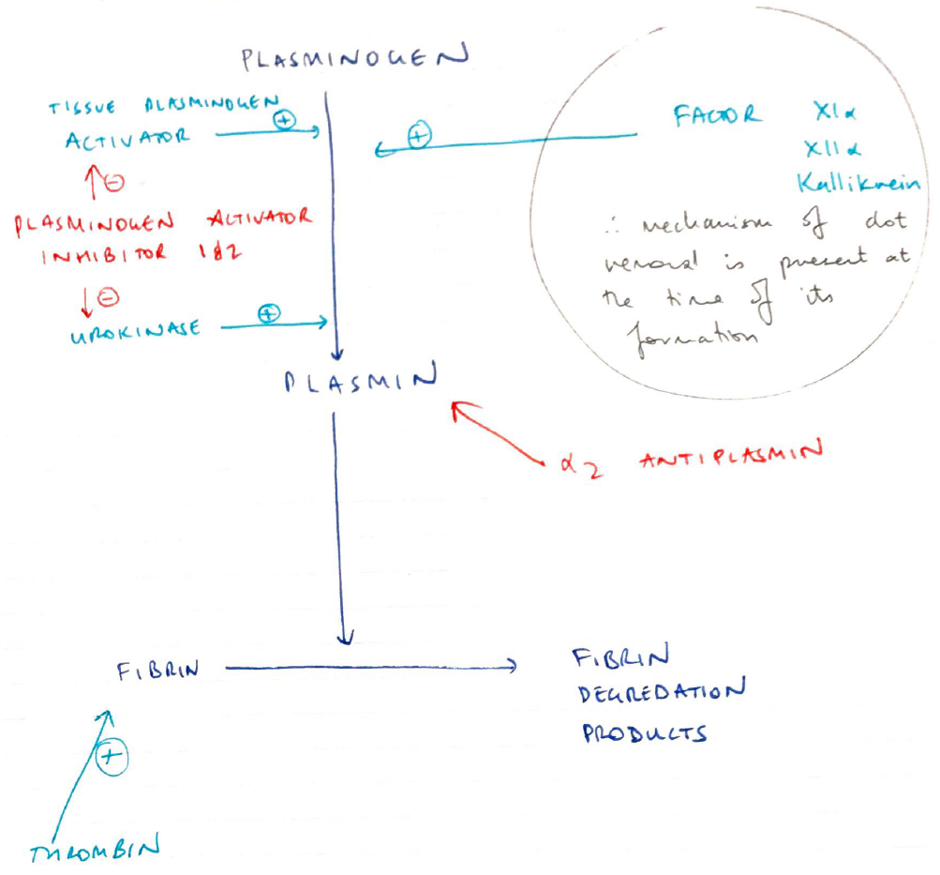
Fibrinolysis
- Contains clot formation
- Both fibrin & fibrinogen bind PLASMINOGEN
- Plasminogen = proenzyme synthesised in the liver
- Plasminogen structured into clot interior
- Plasminogen activators (Urokinase, tPA) are produced by endothelial cells
- Diffuse from Endothelial Cells
- Catalyse PLASMINOGEN → PLASMIN
- Plasmin in circulation metabolised by:
- A2 antiplasmin
- Plasminogen Activator Inhibitor
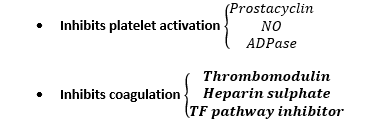
Naturally Occuring Anticoagulants
Tissue Factor Pathway Inhibitor
- Inhibits TF – VIIa/Xa complex downstream from injury
Heparin Sulphate – AT III
- Neutralises free Xa & thrombin
Thrombomodulin
- A protein on Endothelial cells
- Co-factor for thrombin
- But ∆thrombin from PROCOAG → ANTICOAG
- Thrombin bound THROMBOMODULIN → activates Protein C
Protein C
- Anti-inflammatory
- Cytoprotective
- Anticoagulant
- Maintains permeability of blood vessels
Fibrinolytic System
- Any initiation of clotting also activates fibrinolysis
- Plasminogen/plasmin built into clots → preferentially activated by fibrinolysis activators
- Author: Krisoula Zahariou