K1vii / 19A15: NMDA receptor and Ketamine
19A15: Exam Report
Describe the physiology of the NMDA (N-Methyl D-aspartate) receptor (40% of marks). Outline the pharmacology of ketamine (60% of marks).
49% of candidates passed this question.
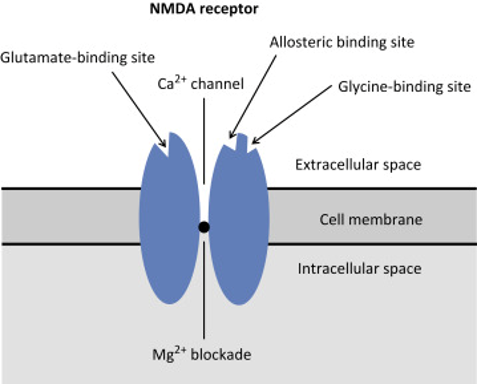
The NMDA receptor is a ligand gated voltage dependent ion channel located on post synaptic membranes throughout the CNS, with glutamate, an excitatory neurotransmitter, its natural ligand. A brief description of its structure, roles of glycine and magnesium, ions conducted, result of activation, role in memory and learning and agonists/antagonists was expected. Detail
on structure and functions of the receptor were a common omission.
Ketamine, a phencyclidine derivative, is a non-competitive antagonist at the NMDA receptor. It is presented as a racemic mixture or as the single S(+) enantiomer (2-3 X potency). Administration routes and doses scored marks. Pharmacodynamics were generally well covered including CVS (direct and indirect effects), CNS (anaesthesia, analgesia, amnesia, delirium, effects on CBF and ICP) respiratory (bronchodilator with preservation of airway reflexes) GIT effects (salivation, N and V). Knowledge of specific pharmacokinetic parameters was less well covered, including low oral bioavailability and protein binding and active metabolite
(norketamine).
K1vii / 19A15: Describe the physiology of the NMDA receptor (40 marks). Outline the pharmacology of Ketamine (60 marks)
Nmda Receptor
Definition: NMDA Receptors are ionotropic glutamate receptors
Located
Exclusively in neurons, widespread and expressed at post synaptic site
Function
Slow ESPS
Growth
Differentiation
Apoptosis
Synaptic Plasticity (Learning & Memory)
Excitotoxicity (ie pathophysiology of epilepsy, Alzheimer’s Disease)
Pain Sensitization:
- Persistent pain will ↑[GLUTAMATE] & ↑signal transmission & causing Mg2+ dislodgement → NMDA receptor activates → CENTRAL SENSITISATION via 2nd messenger & further release of excitatory NT which ↑pain signal, even to benign stimuli
- KA WIND UP
Endogenous Agonists
Glycine
Glutamate/Asparate (not as strong)
Ions Conducted
High Ca++ permeability
Na, K permeability
Result of Activation
- Binding GLUTAMATE
- Binding co-agonist GLYCINE
- Membrane depolarisation (partial by other receptors AMPA & NK on some neurons)
↓
Mg2+ block released
↓
Na+, Ca2+ influx/K+ outflow
↓
Activation of protein kinase & phosphorylating enzymes
Channel Blockers
Phencyclidine
Memantidine
Ketamine
Mg++
Ketamine
Chemical
Phencyclidine derivative, dissociative anaesthetic
Use
- Induction GA
- Conscious sedation
- Analgesia
- Severe asthma
Presentation
2mL vial → 200mg in 2mL, clear colourless solution
- pKa 7.5 → 40% ionised at pH 7.4
- H2O soluble → EXTREMELY LIPID SOLUBLE
ISOMERISM solution contains 50:50
- Ketamine contains an asymmetrical carbon atom with 2 optical isomers (enantiomers)
S (+)
- 3 x potent
- Intense analgesia/anesthesia
- Lower emergence reactions
- More rapid metabolism/recovery
- Less CVS stimulation
R (-) = more bronchodilation & psych SE
Dose
ANALGESIA: 0.1 – 0.2mg IV bolus → then 0.1 – 0.3mg/kg/hr thereafter
SEDATION: 0.3 – 0.5mg IV
GA: 1 – 2mg/kg IV
Route
IV/IM/PO/PR
↓
But poor OBA 16% due to high 1st pass metabolism
Onset
30 – 60sec IV → 3 – 8mins IM
DoA
Return of consciousness
10 – 20mins
MoA
- NMDA Antagonism
- Voltage sensitive Ca2+ channel inhibition
- Muscarinic antagonist
- Facilitates descending inhibitory monoaminergic pathways → inhibits reuptake of CA → INDIRECT SYMPATHOMIMETIC
- Weak opioid receptor agonism
PD
CNS
- Dissociative anaesthesia with slow nystagmic gaze
- Dissociates thalamus (relays sensory info from RAS to C. Cortex) from Limbic Cortex (awareness & sensation)
- Amnesia → anterograde
- Analgesia
- Emergence delirium → visual, auditory, illusion & delirium
- Cerebral protection → ↑CMRO2 & ↑CBF
(Directly dilates cerebral arteries)
RESP
- ↑airway secretions → anticholinergic
- Preserves laryngeal reflexes
- Bronchodilation → ↑symp & Ca2+ channel inhibition
- ↑PAP → ↑symp NS
CVS
- ↑MAP, PAP, CVP, HR, CO, myocardial O2 requirements
- Actually a Direct – ve Inotrope
- But with ↑symp NS & adequate catecholamine stores, therefore CVS stimulating effects predominate
NB: can be unmasked in ICU patients with ↓catecholamine stores
GI
- Salivation = antimuscarinic
- ↑BSL = ↑symp
PK
A
Lipid soluble → absorbed, but poor poor OBA 16%
Due to high 1st pass metabolism
D
- Large → 5L/kg
- PPB small 12%
- Rapidly crosses placenta
- *HIGH LIPID SOLUBILITY *
- Peak plasma 1 min post IV
↓
Rapidly distributed to VRG
Extreme lipid solubility & ↑CBF = rapid crosses BBB & ↑brain [ ]
↓
Subsequently redistributed from brain to less well perfused tissues
M
High hepatic ER
- Clearance 18mL/kg/min
- ∆HBF will alter clearance
- DE-METHYLATION by CYP450 to NORKETAMINE (25% potency)
↓
HYDROXYLATION & CONJUGATION to inactive & glucuronide metabolites
E
H2O soluble metabolites excreted by kidney
<5% unchanged
Faecal excretion <5% dose
Adverse Effects
Tolerance (enzyme induction
IHD/HTN/CCF = Increases myocardial work
Increases ICP, CBF and CMRO2
Pregnancy = reduces uterine BF
Prolongs SUX activity
Unmasks myocardial depression when given w sympatholutic
Enhances NDMR block
- Author: Krisoula Zahariou