F8i / 23A13 / 20A11: Describe the structure and function of adult haemoglobin
23A13: Exam Report
Describe the structure and function of adult hemoglobin
25% of candidates passed this question.
Good answers provided detail about the specific composition of haemoglobin and related the pertinent features of the molecule to its functions in the carriage of oxygen, carbon dioxide and its role in acid-base balance, including the appropriate mechanistic description.
Many candidates provided information on the production and breakdown of haemoglobin which was not required. Many candidates provided an unnecessary amount of detail and diagrams of the oxygen haemoglobin dissociation curve which did not score additional marks.
20A11: Exam Report
Describe the structure and function of adult haemoglobin.
57% of candidates passed this question.
Marks were awarded for the two components of this question – structure and function. The structure component was often only briefly described with a cursory overview provided; however, this component contributed around half of the available marks. Many candidates were unable to accurately describe the structural components of the haemoglobin molecule. The functional component was handled better – however much time was wasted with detailed drawings of the oxyhemoglobin curve (not many marks awarded for this). The basic function of haemoglobin carriage of oxygen and carbon dioxide was known, but detail was often missing about its role as a buffer or its role in the metabolism of nitric oxide.
F8i / 23A13 / 20A11: Describe the structure and function of adult haemoglobin
Definition
- A molecule of 4 protein chains, each carrying a heme group: MV 65,000 Daltons
- Each RBC consists of 200 – 300 million molecules of Hb
Structure
- 2 parts: heme + globin
- Each heme moiety consists of PROTOPORPHYRIN RING & central iron ion in ferrous state (Fe2+)
- Heme synthesis occurs in RBC cytosol & mitochondria
- Fe2+ forms 6 bonds with heme moiety; 5 bind Fe2+ firmly, 1 binds O2
- 4 polypeptide chains determine type of Hb
- HbA = 2α + 2β globin chains
- HbF = 2α + 2γ globin chains
- HbA2 = 2α + 2δ globin chains
- Altered polypeptide chains create variants important to know because alter body’s capacity to transport O2
NB: 98% of normal adult Hb is HbA
Function
- Transport O2 from lungs → tissues
- Transport CO2 from tissues → lungs as CARBAMINO Hb
- Buffer H+ formed in RBC
- NO metabolism
1. Transport O2 from Lungs → Tissues
- O2 binds reversibly to heme
- 4 hemes → each Hb can carry 4 x O2
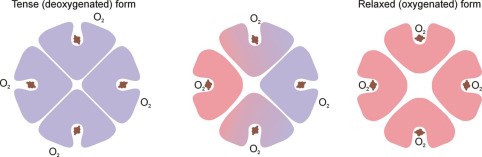
- Hb is an allosteric protein
- The binding of O2 to one heme group ↑affinity of remaining heme groups for O2
- This +ve cooperativity means that DeoxyHb & oxyHb have very different structures
- This shape is maintained by electrostatic bonds b/w α-acid sequences
- DeoxyHb: electrostatic bonds are strong, hiding heme deep in crevice, making it difficult for O2 to access heme this quaternary structure → KA TENSE FORM
- OxyHb = binding of first O2 to a heme changes the electrostatic bonds. Relaxes the crevice holding heme and enlarges the access → transmits this to other globin chains by distracting their electrostatic bonds & the quaternary structure is altered
- ↑ other globin affinity for O2
- KA RELAXED FORM when 4O2 bound to 1Hb
- Huffner Constant
- O2 binding capacity of Hb = amount of O2 in mL each Hb can carry
- KA HUFFNER’S CONSTANT = 1.3mL per 1g of Hb
- The conformational state of Hb (R/T) is also altered by other factors, which influence strength of electrostatic bond:
- CO2
- pH
- Temperature
- The Oxyhaemoglobin Dissociation Curve
- O2 binds reversible with ferrous iron of Hb to form oxyhaemoglobin
Hb + O2 ⮂ HbO2
- Forward reaction occurs in lungs due to ↑PO2
- Backward reaction occurs in tissues due to ↓PO2
- Curve is SIGMOID SHAPE due to COOPERATIVITY
- Affinity for O2 is lowest when first molecule binds to ‘TENSE’ deoxygenated Hb
- As each subsequent molecule binds, the gradient of the curve increases
- As PO2 increases, all the Hb – O2 binding sites become occupied & the curve levels off
- 3 important points on ODC:
- Arterial PO2 where Hb 100% saturated (PO2 100 = SpO2 97%)
- Venous saturation (PO2 40 = SpO2 75%)
- P50 (P50 = 27mmHg PO2 = SpO2 50%)
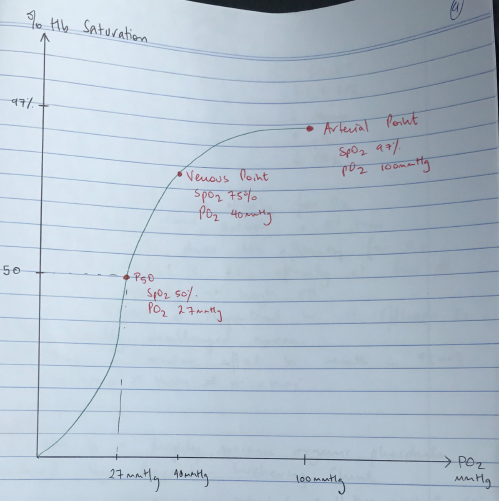
- P50 = the partial pressure of oxygen in blood where Hb is 50% saturated
- P50 is a measure of oxygen affinity
- ∴It is useful to compare changes in the position of the ODC
- P50 for any single form of Hb is variable → the hydrogen bonds & ionic interactions within Hb result in altered affinity of Hb for O2
R) Shift
↑2,3 DPG
↑Temp
↓pH
↑CO2
L) Shift
↓2,3 DPG
↓Temp
↑pH
↓CO2
pH
- An ↑[H+] will ↓Hb affinity for O2
- DeoxyHb binds more actively with H+ ions
- ∴as pH ↓so does Hb affinity for O2
KA BOHR EFFECT: ↓ O1 affinity at low pH & ↑ O2 affinity at high pH
- The Bohr Effect is important because it offloads O2 to metabolically challenged areas
- CO2 has a similar effect because results in ↑[H+]
CO2 + H2O ⮂ H2CO3 ⮂ H + + HCO3–
2, 3 DPG
- 2,3 DPG is a highly anionic organic phosphate
- Produced by Rapport – Luebering Shunt
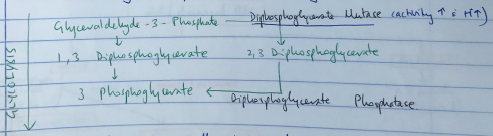
- This shunt comes off the glycolysis pathway
- Level of 2,3 DPG determined by balance of synthesis/degradation
- Activity of DPG Mutase ↑ with ↑H+
- ∴↓Hb affinity for oxygen & displacing ODC to RIGHT
- 2,3 DPG is ↑in ANAEMIA & HIGH ALTITUDE
- ∴tissue hypoxia of anaemia partially corrected by ↑P50
- The ↑RESP ALKALOSIS at altitude has much more pronounced effect than the ↑2,3 DPG & at high altitude ODC shifts LEFT
2. Transport CO2 from Tissues → Lungs as CARBAMINO Hb
- 6% of CO2 in blood is as Carbamino Compounds
- Carbamino compounds are formed when CO2 binds to Hb
- Side chain of valine residues have an N-terminal amino group to which the CO2 will bind to form carbamates
- The carbamate stabilizes the Hb in the Tense Form
- This lowers to affinity of Hb for O2 (KA Haldane Effect)
- Carbamino formation accounts for 2/3 of the Haldane Effect
3. Buffer H+ formed in RBC
- Buffer = a substance with the capacity to bind/release H+ to minimise pH ∆
- Hb is an Intracellular buffer because exists inside RBC but classified as an extracellular buffer because buffers extracellular acids rapidly
- Exists in RBC as weak acid (HHb) & its potassium salt (KHb)
- Buffers via the imidazole residues of histidine residues with pKa 6.8
- Each Hb has 38 histidine residues – large buffering capacity
- Hb is present in high concentrations → 15g/dL
- DeoxyHb kPa = 7.9
- OxyHb kPa = 6.8
- When Hb is deoxygenated → conformational ∆ alters pKa of histidine residue & makes it more effective buffer at physiological pH → this makes up the other 1/3 of the HALDANE EFFECT (deoxygenated blood can carry more CO2)
- Hb will also buffer by binding CO2 to form carbamino compounds
Clinical Importance
- Buffers H+ & CO2 from cellular metabolism
- Buffers fixed acids prior to renal excretion
- Assists CO2 carriage in blood
- Conformational ∆ to deoxyHb
- 7 mmol H+ can be taken up
- ∴7 mmol CO2 enters venous blood with minimal pH Δ
Reactions in RBC
- Cell metabolism produces CO2
- CO2 diffuses into RBC
- CO2 + H2O ⮂ (Ca) H2CO3 ⮂ H+ + HCO3–
- H+ taken up by histidine residues → buffered
- HCO3– exchanged with Cl– via antiporter → HAMBURG EFFECT
- Some CO2 binds terminal amino groups → CARBAMINO COMPOUNDS
4. No metabolism
- NO is produced by endothelial cells by NO synthase
- NO is highly vasoactive:
- Vasodilates smooth muscle
- Inhibits platelet activation
- Induces expression of endothelial adhesion molecule
- Its rapid reaction with Hb is the major mechanism for disarming its bioactivity:
- HbO2 + NO → MetHb + NO3–
This forms methemoglobin (in which heme irons are ferric) and nitrate
- Preservation of NO activity by Hb occurs when deoxygenated hemes form iron-nitrosyl Hb:
- Hb + NO → HbNO
This reaction can occur at any of the 4 hemes when they are deoxygenated
The rate of this reaction is extremely slow, so this ‘capturing’ of NO is not an effective way to transduce NO bioactivity
- Author: Krisoula Zahariou