J1iii / 21A11: Describe the buffer systems in the body
21A11: Exam Report
Describe the buffer systems in the body
57% of candidates passed this question.
This is a core physiology topic; a detailed knowledge of buffering and the available buffer systems is crucial to ICU practice.
A candidate presenting for the first part exam should have a detailed understanding of all aspects of the buffer systems. Higher scoring answers provided both technical details of the buffer systems, the context for their normal function and their relative importance. Efficient answers dealt with the buffers by chemical rather than by site, but many answers categorising buffers by site also scored well. Many low scoring answers simply failed to provide detail, some provided incorrect information. Very few candidates demonstrated an understanding of the isohydric principle.
J1iii / 21A11: Describe the buffer systems in the body
Name:
Bicarbonate-carbonic acid buffer
Location:
Extracellular fluid: RBC, Plasma, ISF
Function
- 80% buffering capacity
- Low pKA (6.1)
- Relatively small amounts in ECF ↓ its effectiveness as a buffer system, but this is offset by being an open system
- Can be controlled independently by
- Lung: Excretion of CO2 (or H2CO3) regulated rapidly by changes in minute ventilation
- Kidney: Excretion of HCO3- and H+ regulated more slowly
Name:
Haemoglobin
Location:
Major non-HCO3– buffer in ECF
Function
Mechanism for buffering capacity of Hb:
- (1) ↑ [Hb] within RBCs (150 g/L) → Large amount of buffer present
- (2) Structure of Hb
- (a) Multiple (38) Histidine residues with Imidazole side chain (pKa 6.8) on globin chains of Hb → Anionic sites on Imidazole binds H+ → Contributes to MAIN buffering capacity of Hb at physiological pH
- (b) Amino acid groups on globin chains of Hb → CO2 and H2CO3 can bind with terminal amino groups of amino acids of Hb to form “carbamino compounds”
HbNH2 + CO2 → HbNHCOO– + H+
HbNH2 + H2CO3 → HbNH3 + HCO3–
- (3) Hb is a weak acid
- Hb exists as weak acid (HHb) and salt (KHb) with a pKa 6.8 → it is an even weaker acid cf. HCO3–H2CO3 buffer system (pKa 6.1)
- Thus, in presence of ECF acid → Hb buffers extracellular H+ → leads to HCO3- formation within RBC → HCO3- accumulates and diffuses down its electrochemical gradient out of RBC → ↑ plasma HCO3-
- (4) RBC contains carbonic anhydrase and has ↑ solubility for CO2:
- Hb contributes to > 90% of blood capacity to buffer CO2 (as H2CO3)
- Tissue CO2 diffuses into RBC whereby it is hydrated to form H2CO3 by CA → (i) H+ produced then preferentially binds DeoxyHb (to form Reduced Hb), thus allowing offloading of O2 to tissues, and (ii) HCO3- produced diffuses down its electrochemical gradient out of RBC → ↑ plasma HCO3-
- (5) DeoxyHb is a better buffer than OxyHb (Haldane Effect)
- DeoxyHb (pKa 8.2) is a better buffer than OxyHb (pKa 6.6) because it is a weaker acid and dissociates to a greater extent
- 6x more buffering capacity than plasma protein
- Higher protein concentration
- X3 buffering capacity cf plasma protein due to x3 more imidazole containing histidine residues
Name:
Phosphate buffer
Location:
Urine
intracellular
Function
Phosphoric acid is a tribasic acid:
H3PO4 ↔ H+ + H2PO4 (pKa 2)
H2P04 ↔H+ + HPO42- (pKa 6.8)
HPO4– ↔ H+ + PO43- (pKa 11.7)
Main buffer intracellularly and in urine because:
- [ ] are much ↑
- intracellular and urine pH are more acidic (Ie. closer to pKa of phosphate buffers)
- pKa 6.8
- closed system
- Important in “bone buffering” → CaPO4 acts as “Alkali reserve” → During prolonged acidosis, it solubilises in plasma to ↑ [PO43]
Name:
Ammonia
Location:
Urine
Function
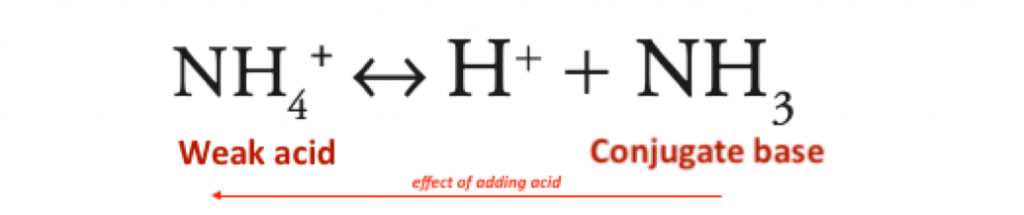
Ammonium = protonated form of ammonia; it is an acid because it contains a dissociable proton
- High pKa 9.1 (extremely weak acid)
- an extremely weak acid – pKa 9.2
- therefore in the renal tubule it is largely in the NH4+ form
- causing excretion of H+
- urine pH
- normal = 6.0
- minumum = 4.4
- Open system, however far slower to accommodate changes than lungs
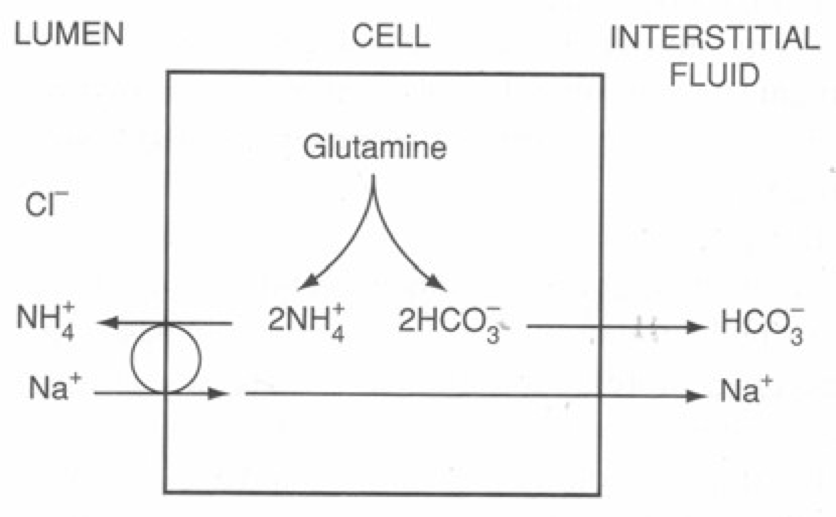
- Ammoniagenesis → PCT tubular cells produce NH3:
- Filtered glutamine is taken up by PCT tubular cells and deaminated via Glutaminase (which is upregulated with chronic acidosis) into NH4+ and HCO3–
- NH4+ is secreted into the tubular lumen via a Na+/ NH4+ antiport, while “newly formed” HCO3– is reabsorbed into peritubular capillary blood
- Ammonium cycling → In the TAL of LOH, 80% of tubular NH4+ is reabsorbed → this generates ↑ [ ] gradient of NH4+ in the medullary interstitium
- Ammonium excretion → Later in the medullary CD:
- NH3 diffuses from medullary interstitium into tubular cells of the CD, then into tubular fluid
- Within the tubular cells of the CD, H+ and HCO3– is formed by hydration of CO2 using intracellular CA
- H+ secreted into tubular fluid by Na+/H+ antiport → combines with tubular NH3 to form NH4+ → “ion trapped” in tubular fluid
Author: Freddie Hopkinson / Huiling Tan