F5i / 22B15: Describe the anatomical (20% marks) and physiological (80% marks) features of the pulmonary circulation
20B15: Exam Report
Describe the anatomical (20% marks) and physiological (80% marks) features of the pulmonary circulation
23% of candidates passed this question.
Many candidates described the anatomical pattern of right ventricle to arteries to smaller arteries to arterioles to capillaries to venules to veins to the left atrium.
To obtain full credit one needed to describe relevant aspects of anatomy including main pulmonary artery ~5cm in length divides into L and R pulmonary arteries.
Arteries are relatively thin walled with little smooth muscle, capillaries form an extensive sheet of blood flow over the alveolar wall, and the pulmonary circulation drains into four pulmonary veins that empty into the left atrium.
A structured approach would then follow regarding physiology. There is ~500 ml of blood in the pulmonary circulation with ~10% in the capillaries and half the remainder in each of arteries and veins.
The system has a high capacitance and is very distensible.
The volume can halve or double to adjust for posture, respiratory effort and changes to the systemic circulation.
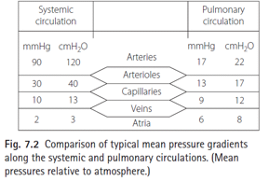
Values for normal pulmonary artery pressure were expected and an explanation that this is just adequate to reach the apices of the lung and that if the pulmonary pressure was higher there would be a risk of compromised perfusion and flow.
Comparisons with the systemic pressures or detail such as capillary values gained extra credit. For example, highlighting that regional distribution and regulation is relatively passive compared to the systemic circulation and thus gravity and posture have significant effects.
Many candidates gave the units incorrectly. Of the candidates who described the West Zones, most seemed aware of the influence of alveolar pressure, but few seemed aware of the importance of low pulmonary pressure relative to the effect of gravity, such that the pulmonary arterial pressure was just adequate to reach the apex of the lung.
Most candidates were aware of the important role of hypoxic pulmonary vasoconstriction to optimise VQ matching.
The autonomic system has relatively little effect upon regulation especially compared to the systemic circulation.
Many candidates wrote in generalities about the physiology of circulatory systems without discussing special features of the pulmonary circulation.
The detail supplied was often less than the expected level.
F5i / 22B15: Describe the anatomical (20% marks) and physiological (80% marks) features of the pulmonary circulation
The pulmonary circulation is a low pressure, high capacitance system with the main function being the oxygenation of blood and removal of carbon dioxide.
Anatomy
Pulmonary circulation and travels in a unilateral direction beginning at the right ventricle:
- Main pulmonary artery ~ 5 cm in length
- Left and right pulmonary arteries
- Smaller arteries – relatively thin-walled with little smooth muscle
- Arterioles
- Capillaries – form an extensive sheet of blood flow over the alveolar wall
- Venules
- Veins – become four pulmonary veins
- Oxygenated blood returns to the left atrium
Physiology
Pulmonary blood volume
500 ml of blood in circulation – 10% in capillaries, 45% each in arteries and veins
Volume can halve or double to adjust for:
- Posture (supine to erect decreases blood volume by almost 1/3 due to pooling of blood in dependent regions)
- Respiratory effort
- Changes to the systemic circulation (increase in systemic vascular tone will squeeze blood into the pulmonary circulation
Blood flow
Approximately equal to cardiac output, 6L/min in resting conditions and up to 25L/min in severe exercise (achieved with minimal increase in pressure)
Limited ability to control regional distribution of blood flow (hypoxic pulmonary vasoconstriction) results in overperfusion of dependent parts of the lung fields.
Pressure
Pressure of the capillary bed will also vary depending on the zones of the lungs (8 in the upper, 22 in the middle and 35 in lower zones)
Vascular resistance
PVR = Pulmonary driving pressure/Cardiac output = (mPAP – mLAP)/CO
144 dynes.s.cm-5 (Nunn’s)
Resistance equally divided between arteries (mostly extra-alveolar and under nervous, humoral or gaseous control), capillaries (alveolar, influenced by alveolar pressure and volume) and veins.
- As opposed to most of the resistance occurring in arterioles in the systemic circulation
Passive changes to PVR occur partly by passive distension of vessels and partly by recruitment of collapsed vessels, both reducing PVR.
- Recruitment of previously un-perfused pulmonary vessels occurs in response to increased pulmonary flow. Greatest effect at the capillaries lacking muscle and most likely to occur in West Zone 1.
- Distension in the entire pulmonary vasculature occurs in response to increased transmural pressure gradient. Most likely to occur in capillaries
PVR lowest at FRC and increases when lung volume changes from FRC.

(Compression of alveolar capillaries – dashed line, compression of extra-alveolar vessels at low lung volumes or hypoxic pulmonary vasoconstriction – dotted line)
West’s Zones
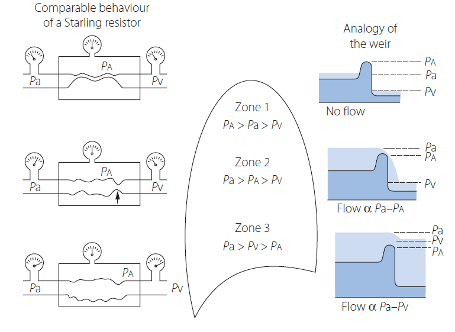
Lung is divided into zones with relative differences in interplay of alveolar pressure, flow rate and vascular resistance.
Zone 1 – Alveolar > arterial pressures, insufficient to open vessels that remain collapsed. – Corresponds to the apex of the lungs.
Zone 2 – Pressure at arterial end of the collapsible vessels exceeds the alveolar pressure. Performs as a Starling resistor, permits flow in such a way that the flow rate depends on the arterial/alveolar pressure difference.
- The greater the difference between arterial and alveolar pressures, the more widely the collapsible vessels will open and greater the flow. Venous pressure is not taken into account for flow through the vessel.
Zone 3 – Pressure in the venous end of the capillaries is above alveolar pressure. Vessel held open and flow rate will be governed by arterial/venous pressure difference (driving pressure)
- Corresponds to dependent/bases of the lungs
Vascular resistance (active control)
Adrenergic:
- a1 – constriction (noradrenaline) – dominant
- a2 – dilatation (noradrenaline)
- b2 – dilatation (adrenaline)
Cholinergic:
- M3 – dilatation (acetylcholine), parasympathetic system
Amines:
- H1 – variable (histamine)
- H2 – dilatation (histamine)
- 5-HT1 – variable (5-HT)
Purines:
- A1 – constriction (adenosine)
- A2 (dilatation (adenosine)
Eiocosanoids:
- TP – constriction (thromboxane A2)
Peptides:
- NK1 – dilatation (Substance P)
- NK2 – constriction (Neurokinin A)
- AT – constriction (Angiotensin)
ANP – dilatation (ANP) - B2 – dilatation (bradykinin)
- ETA – constriction (endothelin)
- V1 – diltation (vasopressin)
PVR (NO)
Nitric oxide, produced by nitric oxide synthase (converting L-arginine to L-citrulline, via a highly reactive hydroxy-arginine intermediate.
- Reaction requires many cofactors including calmodulin and NADPH.
- Control of NOS activity depends on the availability of the substrate, arginine and concentrations of the various cofactors.
- Citrulline produced by NOS enters the urea cycle and is converted back into arginine. Pathway utilises ammonia derived from conversion of AA into energy-producing substrates such as pyruvate and provides a mechanism by which ammonium ions may be converted into relatively harmess nitrates (via NO)
- NOS exists in 2 forms: constitutive (cNOS is permanently present in some cells, including pulmonary endothelium and produces short bursts of low levels of NO in response to changes in calcium and calmodulin) and inducible (iNOS produced in many cells but only in response to activation by inflammatory mediators and other cytokines). iNOS, once activated, produces large amounts of NO for long periods.
- Basal production of NO occurs in normal human lungs and contributes to the maintenance of low pulmonary vascular resistance (activation of guanylate cyclase converting GTP to cGMP, then reducing calcium and inducing relaxation).
PVR (Hypoxic pulmonary vasoconstriction)
- Mediated by both mixed venous (pulmonary arterial) and alveolar PO2 (greater influence).
- Regional hypoxic pulmonary vasoconstriction is beneficial as a means of diverting the pulmonary blood flow away from regions in which the oxygen tension is low and optimises V/Q relationships.
- Occurs in constriction of small arterioles of 30-200 micrometers and begins within seconds of the PO2 decreasing.
- Within 5 minutes, regional blood flow is half that during normoxia
- HPV is biphasic in prolonged HPV -> initial rapid response reaches a plateau after ~ 5 minutes with a second phase occurring 40 minutes later (peak response at 2-4 hours after onset of hypoxia.
- Mechanism – occurs with contraction of pulmonary artery smooth muscle cells in response to hypoxia. Detection of hypoxia is similar to that of glomus cells in the carotid body with hypoxia leading to a small increase in intracellular concentration, resulting from opening of L-type calcium channels stimulated by hypoxia-induced inhibition of VG potassium channels (Kv).
- Inhibitors of HPV – PGI2, NO
- Enhancers of HPV – thromboxane A2, endothelin
PVR (other factors)
- Hypercapnoea – increases PVR
- Acidosis (resp/metabolic) augment hypoxic pulmonary vasoconstriction
Functions
- Gas Exchange
- Reservoir of blood
- Filter for the blood stream
- Thermoregulation
Author: Michael Wu