J2i / 23A01: Outline the abnormalities in the following arterial blood gas (25% of Marks). Explain the Stewart approach to acid-base interpretation (75% of Marks)
23A01: Exam Report
Outline the abnormalities in the following arterial blood gas (25% of Marks). Explain the Stewart approach to acid-base interpretation (75% of Marks)
21% of candidates passed this question.
High performing answers correctly outlined the ABG findings including consideration of electrolyte abnormalities, A-a gradient, acid-base disturbance (including anion gap and strong ion difference) and whether compensation was appropriate.
The best explanations of the Stewart approach described its physicochemical basis, discussed the independent variables (strong ions, total weak acids, and pCO2) in detail, and described their effect on the dependent variables and how they result in acid-base derangements.
The ABG provided depicted an incorrect base excess with an omission of (-) symbol.
Candidates were marked accordingly depending on their response to this and all candidates were compensated equally for the confusion that this may have caused.
J2i / 23A01: Outline the abnormalities in the following arterial blood gas (25% of Marks). Explain the Stewart approach to acid-base interpretation (75% of Marks)
Interpretation of ABG
An approach to ABG analysis….(find an approach and stick to it – I like Deranged’s)
This is an example ABG and not the ABG on the actual exam paper
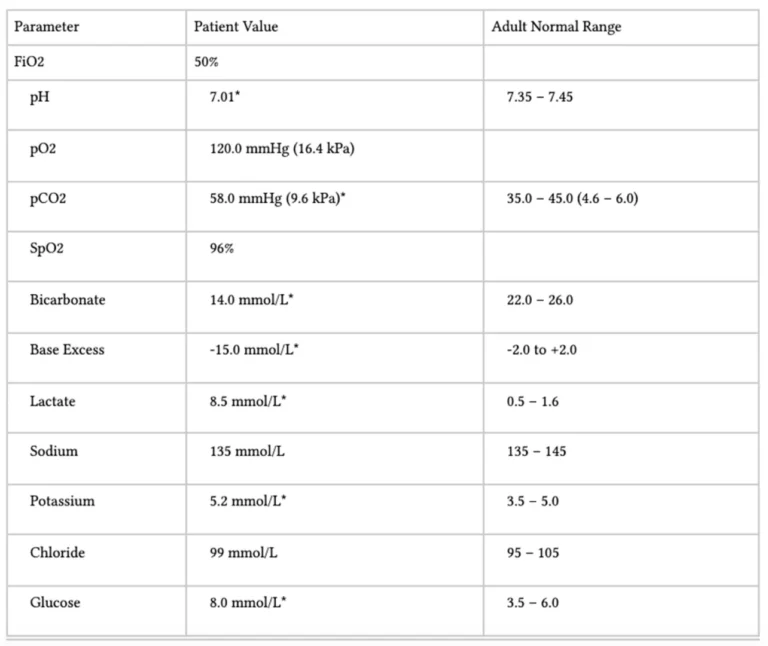
1. A-a gradient: calculate
- PAO2 = (FiO2 x 713) – (PaCO2 x 1.25)
- (expected) PAO2 = (0.5 x 713) – (58 x 1.25) = 356 – 72.5 = 283
- (actual) PaO2 = 120
- A-a = 283 – 120 = 163
- Normal A-a gradient = 10mmHgh +1mmHg for every decade of life
- Therefore there is a raised A-a gradient (you need to show the working to gain marks)
2. Describe the pH change:
- Acidaemia / Alkalaemia
3. What is the Base Excess Change:
HCO3
- 1mmol increase w Acute Respiratory Acidosis
- 4mmol increase w Chronic Respiratory Acidosis
- 2mmol decrease w Acute Respiratory Alkalosis
- 5mmol decrease w Acute Respiratory Alkalosis
CO2
- For Metabolic Acidosis: PaCO2 = (1.5 x HCO3) + 8
- For Metabolic Alkalosis: PaCO2 = (0.7 x HCO3) + 20
Metabolic Acidosis
- ePaCO2 = (1.5 x HCO3) + 8
- ePaCO2 = (1.5 x 14) + 8
- ePaCO2 = 29
- aPaCO2 = 58
- Therefore there is inadequate compensation and a Respiratory Acidosis as well
5. Anion Gap:
- Calculated to evaluate the cause for metabolic acidosis & determine the presence of unmeasured anions
- Normal AG = 4-12
NAGMA (USED CRAP)
- Ureteric Diversion
- SB fistula
- Extra Chloride
- DKA
- CA Inhibitors
- Addisons
- RTA 1,2,4
- Pancreatic Fistula
HAGMA (L TKR)
- Lactate
- Toxins
- Ketones
- Renal (failure to excrete organic anions Sulphate, Hippurate, Phosphate)
- AG = Na – (Cl + HCO3)
- AG = 22
- Therefore there is a raised Anion Gap Metabolic Acidosis
6. Mention other glaring abnormalities
- Make a comment on the electrolytes/glucose/albumin etc if they are given
- Hyperlactaemia which is contributing to the HAGMA
Stewart Approach
Introduction
- AKA Physiochemical Approach
- Differs from HH which is determined by H+ & HCO3–
- 3 independent variables which control acidity
Independent Variables → these ∆ pH (= 1° cause)
- pCO2
- ATOT (total weak non-volatile acids)
- SID
Dependent Variables (= 2° effect)
- These values depend on values of independent variables
- If these ∆, then independent variables must have ∆
- H+
- OH–
- CO32-
- HA (weak acid)
- A– (weak anions)
Physical Laws Of Physiochemical Methods
- Interaction of dependent & independent variables must obey the laws of aqueous solution:
- ELECTRONEUTRALITY – in any aqueous solution the sum of all +vely charged ions must equal the sum of all -vely charged ions
- DISSOCIATION EQUILIBRIA – derived from Law of Mass Action. The velocity of a chemical reaction is proportional to the active [ ] of the reaction
- CONSERVATION OF MASS – the amount of substance remains constant unless added, removed, generated, or destroyed
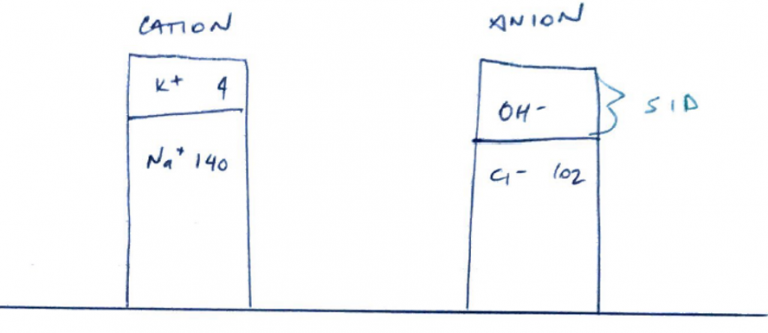
SID in Plasma
- All solutions of body have H2O
- [H+] of these solutions depends on the dissociation of H2O
- H2O dissociation depends on the VARIABLES!
- SID, PCO2, ATOT
- Strong cations = Na, K, Ca, Mg
- Strong anions = Cl, SO42-
- SID = strong cations – strong anions
- Normal plasma SID = 42 mEq/L
- But you must have Electroneutrality
- Whereby Anions = cations so that the SID = 0
- But it’s not, it’s 42mEq/L and slightly alkaline
- ∴somewhere in plasma is unmeasured anions (the OH- in the above gamblegram)
- ↑SID = alkalosis (the unmeasured anions have increased further)
- ↓SID = acidosis (there are less unmeasured anions)
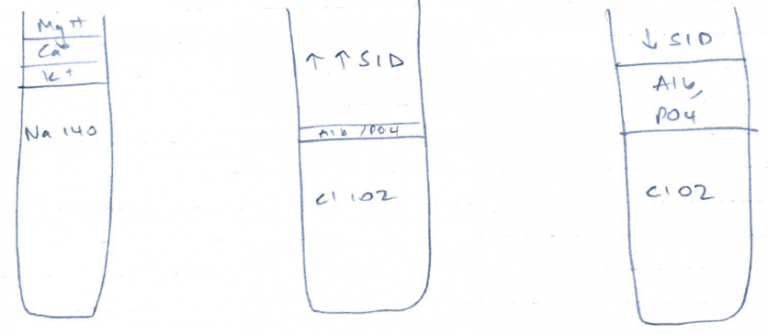
(see Gamblegram)
- ↑/↓ Na
- ↑/↓ Cl –
- ↑ organic acids with pKa < 4 (Strong acids)e. lactate, ketones
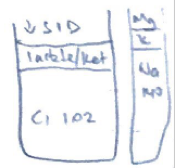
- ATOT
- Non-volatile weak acids (non-CO2) exist in all body fluid compartments
- In plasma: PO42-, serum proteins, albumin
- Controlled by metabolic state & liver
- Non-volatile weak acids dissociate:
HA ↔ H+ + A–
- Total concentration of weak acid = ATOT
- ↑Alb/PO4 = ↓ SID = acidosis
- ↓Alb/PO4 = ↑ SID = alkalosis
- PCO2
- Controlled by respiratory system
- ↑CO2 = ↑H+ (adding)
Classification According to Stewart’s
Resp Causes
- ↑/↓ PCO2
Non-Resp Causes
- ABNORMAL SID ↑/↓ H2O
Water excess = ↓SID
Water deficit = ↑ SID
- STRONG ION IMBALANCE ↓/↑ SID
↑Cl = ↓SID
↓Cl = ↑SID
Anion excess = ↓SID
- ATOT ABNORMALITY ∆ ATOT
↓/↑ Phosphate
↑/↓ Albumin