K3i / 23A09 / 19B12: Pain detection
23A09: Exam Report
Define pain (10% of Marks). Describe how pain is detected and modulated in response to a peripheral noxious stimulus? (90% of Marks)
26% of candidates passed this question.
Candidates were expected to give a reasonable definition of pain incorporating the experience and tissue damage. Most candidates only partially incorporated both the actual or perceived harm and the sensory and emotional experience that is included in its formal definition.
This question was then best answered by breaking down pain transmission and modulation into; peripheral, spinal cord, cortex, and central downregulation pathways. Good answers provided detailed and specific descriptions of the sensors, neural pathways, synapses, receptors, and neurotransmitters. Whilst pain transmission at the level of the spinal cord is complex, breaking this down into 1st and 2nd order neurons, main neurotransmitters and accessory neurotransmitters from interneurons and descending pathways was helpful.
Whilst many covered some of this conceptually most answers did not provide sufficient detail to be considered a pass level answer. Many candidates described the withdrawal reflex to pain in detail which was not asked for and therefore did not attract marks.
19B12: Exam Report
Define pain. Outline the processes by which pain is detected in response to a peripheral noxious stimulus.
26% of candidates passed this question.
Starting with the WHO definition of pain, followed by a brief description of the nature of noxious stimuli (thermal, mechanical, chemical) then proceeding to mention the nature of the cutaneous receptors would have been a very good start to this question. Following this, a description of the various substances involved in pain (K, prostaglandins, bradykinin, serotonin, substance P) and outlining the types of nerve fibres involved in pain transmission and how they synapse in the spinal cord and cortex was expected. The presence and nature of the descending inhibitory pathways was mentioned by very few.
K3i / 23A09 / 19B12: Define pain. Outline the processes by which pain is detected in response to a peripheral noxious stimulus
Definitions
- Pain = an unpleasant sensory & emotional experience associated with actual/potential tissue damage
- Nociception = process by which pain is transmitted from nerves → brain
- Acute pain = recent onset & probably limited duration
- Chronic pain = persists beyond time of healing of injury. Frequently no identifiable cause
- Neuropathic pain = initiated or caused by primary lesion or dysfunction in nervous system
- Nociceptors = detection of potential tissue damaging stimuli
- Noxious stimuli = mechanical, chemical, thermal
Nociceptor
- Transduce noxious stimuli (mechanical, thermal, chemical) into an AP → relay to SC
- Free nerve endings
- Cell bodies present in DRG
- Widespread: skin, muscle, connective tissue, vessels, viscera
- C-fibres: no myelin, <2m/sec, slow burning dull pain poorly localised
- Aδ fibres: myelin, 55m/sec, fast sharp pain
- Transmit impulses to CNS
Inflam Mediators
- Release of NTs/neuropeptides by damaged tissue → K+, BK, leukotrienes, 5HT, histamine, Subs P → released by nerve endings → cause oedema & VD
- Nociceptors are activated by mechanical distortion
- Activation causes neuron to reach threshold → Na+ channel open → AP generated → propagates to SC
Drugs
- NSAIDS – COX inhibition → ↓ PGI production & nerve sensitisation
- LAs – frequency-dependent Na+ channel blockage → prevents neuron depolarising
- Capsaicin – depletes NTs in DRG → prevents impulse propagation
Spinal Cord
- Dorsal horn
- 1° afferent Aδ & C fibres terminate in dorsal horn of SC
- Aδ synapses at I & V
- C synapses at II & III
- Synapse onto 2° order neurons which process, input & modulate pain signal (some inhibitory, most excitatory)
- Complex interaction with:
- Afferent fibres
- Interneurons
- Descending fibres
- Other receptors located in SC
- GABA & GLYCINE (inhibitory) located pre & post synaptically
- OPIOID located pre & post synaptically on horsal horn
- NMDA located post synaptically on 2° efferents Br + SC
- α adrenoreceptors
- Huge amount of integration determines what occurs with pain signal
Drugs
- LAs – prevent neuronal cell depolarisation of SC → injected epidural/intrathecally
- Opioids – antagonism of GPC opioid receptors → inactivate AC → ↓cAMP
Inactivation of Ca2+ channel (↓Ca2+ = ↓NT release)
Activation of K+ channel (↑K+, hyperpolarisation, ↓neuronal excitability)
- Clonidine – α2 agonism at SC → ↓NA release from symp n’s (α2 receptor in Dorsal Horn of SC)
- Ketamine (complex interaction with opioid receptor) – non-competitive antagonism of NMDA Rec Ca2+ pore → ↓glutamine release pre-synaptically → ↓NMDA rec activity
Ascending Tracts & Central Processing
- 2° order neurons ascend in 2 tracts to transport nociception to brain
Spinothalamic (discriminates)
- Crosses ML
- Localises stimulus
- Type of pain
Spinoreticular (behavioural)
- Ascends ipsilat to brainstem
- Integrates signals
- Visceral/endocrine effect
- Emotional response to pain
- 2nd order neurons synapse in THALAMIC NUCLEI with 3rd order neurons
- 3rd order neurons project through IC to cerebral cortex (behaviour) & somatosensory cortex (discrimination)
Drugs
- Paracetamol – central COX inhibition & inhibition of central serotonergic pathways
- Tramadol – weak opioid agonist, NA & 5HT reuptake inhibition
- TCAs – NA + TCA reuptake inhibition; NMDA antagonist
Pain Modulation
Descending Pathways
- Inhibit ascending pathways → provide analgesia
- Originate in somatosensory cortex + hypothalamus
- Activation of periaqueductal grey matter (PAG) & Nucleus Raphe Magnus (NRM)
- PAG disinhibits GABA neurons
- NRM releases 5HT which activates inhibitory interneurons
- Descend to medulla + SC
- Inhibit ascending n. signals by
→ Presynaptic inhibition: prevent Ca2+ channel opening & NT release
→ Postsynaptic inhibition: opening of K+ channel causing hyperpolarisation of membrane
- Desc inhib interneurons activate α2 receptors (clonidine)
Peripheral Sensitisation
- Tissue injury → release of NTs/neuropeptides/inflammatory mediators
- COX2 induction → PG synthesis → direct lowering of activation threshold of nociceptors to:
- ↑rated of DC
- ↑DC strength to any stimulus
→ KA 1° HYPERALGESIA
Central Sensitisation
- Modulation of pain signals in SC
- NMDA receptor in DRG responsible
- Glutamate from 1° afferent → binds NMDA Rec → depolarisation → ↑sensitivity to NTs
- Prolonged C fibre input → ↑rate of firing for same stimulus, even after stimulus has stopped
→ Results in ALLODYNIA HYPERALGESIA, SPONTANEOUS PAIN
- Peripheral & central sensitisation in normal part of nociceptive response → but can be abnormal & cause chronic pain once acute healing has stopped
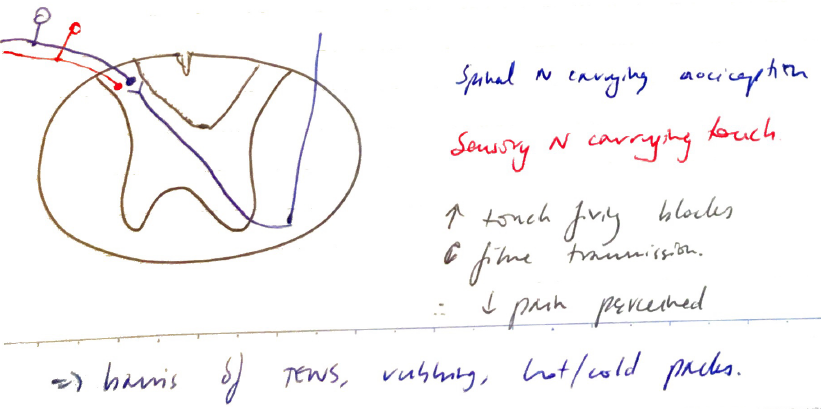
Gate Control Theory
- Can be thought of as ‘ascending inhibition’
- Noxious stimuli are blocked at SC level
- Basis of ‘rubbing a sore’
- Touching the area hurt produces many touch afferent signals
- Enter SC at same level as noxious stimulus
- But laminae can only transmit a certain number of signals
- Overbearing touch signals ↓no of C fibre transmission (carrying pain) through Dorsal Horn
Overview of Pain Pathway
- Noxious stimulus (chemical, thermal, mechanical)
↓
Activates nociceptors (1° afferent neurons)
Aδ = myelin, 55m/sec, fast pain
C = unmyelinated, <2m/sec, slow pain
↓
Activation → threshold → propagate AP → synapse in DORSAL HORN
↓
Release neuropeptides/neurotransmitters
- Injured cells: K, PGI, Leukotrienes
- Glutamate: activates NMDA receptor
- Platelets: serotonin
- MC: histamine
- Subs P: NK
↓
Synapse at 2° order neurons & continues ascent via 2 tracts
1) Spinothalamic = discriminates
- Cross ML
- Localises stimulus
- Type of pain
2) Spinoreticular = behavioural
- Ascends ipsilateral
- Integrates signal
- Emotional response to pain
↓
- Reach THALAMUS, where they synapse with 3rd order neurons
↓
- 3rd ORDER NEURONS project through IC to cerebral & somatosensory cortex