J1iii / 25A04 / 14A22: How do the body’s buffer systems work
25A04: Exam Report
Define a buffer (10% of marks).
Describe the buffer systems of the body (90% of marks).
69% of candidates passed this question.
- A definition of a buffer such as that found in a general physiology textbook such as Power and Kam were required (i.e. “a solution consisting of a weak acid and it’s conjugate base which resists a change in pH when a stronger acid or base is added”).
- The major buffer systems in the body are bicarbonate, haemoglobin, protein, phosphate and ammonia. Full marks were awarded for answers that included a description of each system, their pKa, whether they are ‘open’ or ‘closed’ and their relative importance.
14A22: Exam Report
What is a buffer? (10% of marks)
Discuss the body’s buffer systems and how they work. (90%of the marks)
37% of candidates passed this question.
Candidates who described an overall view of the body’s buffer systems scored well.
Most candidates could define buffer and could discuss the bicarbonate buffer system; however this was not sufficient to pass the question. To pass there had to also be a discussion of the other buffer systems including phosphate, protein (haemoglobin, plasma proteins and intracellular proteins), ammonia and bone.
Best answers included discussion of open versus closed systems and the relative contribution of the differing buffer systems in blood, intracellular fluid, extracellular fluid and urine.
J1iii / 25A04 / 14A22: What is a buffer (10 marks)? Discuss the body’s buffer systems and how they work (40 marks)
- Definition: buffer = substance which has the capacity to bind/release H+ to minimise pH Δ
- Buffer mixtures are a weak acid & its conjugate base
- H2PO4– (weak acid) ↔ HPO42- (conjugate base) + H+
- Buffers are most effective at their pKa, where they are 50% ionised
- AIM: to minimise pH Δ to allow time for correction of 1° acid-base disorder
- Target pH ECF = 4
- Target pH ICF = 8
- Buffer power determined by:
- pKa
- Concentration of the buffer
Buffer Systems Classified
ICF | ECF |
Hb | HCO3– |
Proteins | Hb |
Phosphate | Plasma proteins |
Phosphate |
ECF HCO3–
- pKa 6.1
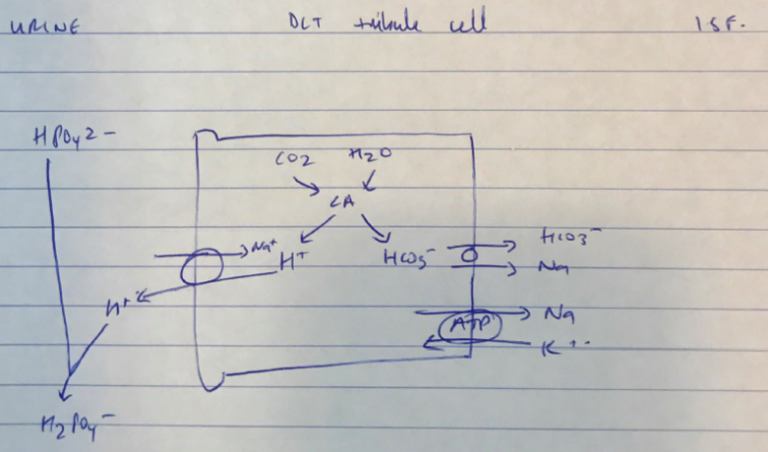
- Concentration: highest with plasma [HCO3–] = 24 mol/L
- HCO3 – production occurs in RBC in presence CA:
- CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3– → diffuses out & buffers ECF
- Although pKa very different from target pH, so effective because:
- High concentration
- Open-ended
- Considered “open ended” because its components; CO2 & HCO3– are regulated independently
- CO2 → lungs → exhaled
- HCO3– → kidneys → reabsorbed
- HCO3– buffer system can only buffer metabolic acids
Hb – ECF/ICF
- pKa 6.8
- Concentration = 15g/dL
- Actually intracellular protein, but because it’s present in ‘blood’, not tissue → can be considered ECF buffer
- Each Hb → 68 histidine residues
- Hb high concentration in plasma
- DeoxyHb = pKa 7.9
- OxyHb = pKa 6.6
- ∴ DeoxyHb dissociates greater at pH 7.4 cf. oxyHb
- H+ taken up by histidine residues → buffered
- Some CO2 binds to terminal amine groups → forms carbamino compounds
ECF – Plasma Proteins
- pKa 6.8 (of imidazole of histidine residues)
- Concentration = 7g / dL
- The histidine residue binds & buffers H+
ICF – Plasma Proteins
- pKa 6.8 → imidazole buffers H+
- Significant concentration 6 mol/L intracellular
- Cell pH 6.8 – 7.1 ∴ closer to imidazole pKa
- ∴ very effective intracellular buffer!
Phosphate – ICF
- pKa 6.8
- High concentration intracellular 6mol/L
- Very close ICF pH 6.8 – 7.1
- ∴ very important ICF buffer with plasma proteins
- Author: Krisoula Zahariou