H1v / 20B19 / 14B12: Kidneys Non Volatile Acid Secretion
20B19: Exam Report
Explain how the kidney handles an acid load
51% of candidates passed this question.
This question required candidates to understand the renal response to an acid load. It was expected that candidates would answer with regard to recycling of bicarbonate in the proximal tubule, excretion of titratable acid via the phosphate buffer system and generation of ammonium and its role in acid secretion. Many candidates had a good understanding of the bicarbonate system but used this to explain the secretion of new acid.
14B12: Exam Report
Describe the role of the kidneys in the excretion of non-volatile acid.
27% of candidates passed this question.
This is a complex but essential area of physiology for intensive care practice. It was expected candidates would indicate that non-volatile acids are those not able to eliminated by the lungs (lactate, sulphate, phosphate and ketone bodies). The kidney plays a central role via bicarbonate (resorbing filtered bicarbonate and generating “new” bicarbonate = acid excretion). It was
expected candidates could detail the processes in various parts of the renal tubules and the role or urinary buffers (dibasic phosphate and ammonia).
While a number of candidates were able to provide some of the details of ion transports in the kidney, few showed understanding of the overarching concepts of the proximal bicarbonate reabsorption being necessary to allow the distal acid excretion.
H1v / 20B19 / 14B12: Describe the role of the kidneys in the excretion of non-volatile acid
Definition
Non-volatile acid: an acid not excreted by lungs
- Fixed acid production = 1 mmol / kg / day
- Due to incomplete metabolism of:
- Carbohydrates → lactate → 1500 mmol/day
- Protein → sulphate, phosphate → 55 mmol/day
- Fat → ketones → 12 mmol/day
- These acids require excretion by the kidneys for acid-base balance (~70 mmol/day)
NB: lactic acid is exempt because 1500mmol of lactic acid produced is oxidised by the liver to regenerate HCO3–
- Kidney has 2 important roles in acid-base & excretion of fixed acids
- Reabsorb all filtered HCO3– (4320 mol/day)
- Excrete fixed acids (70 mmol/day)
1) HCO3- Reabsorption
- Daily acid load cannot be excreted unless all filtered HCO3– is reabsorbed
- Because both derived from H2CO3 dissociation
- H2CO3 ⮂ H+ + HCO3–
- Losing HCO3– is like gaining H+
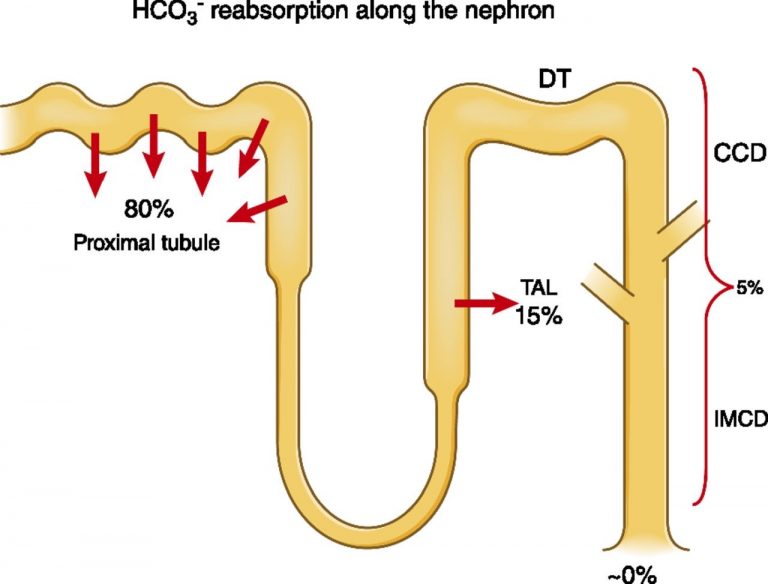
- HCO3– reabsorption
- 80% PCT
- 15% thick ascending LoH
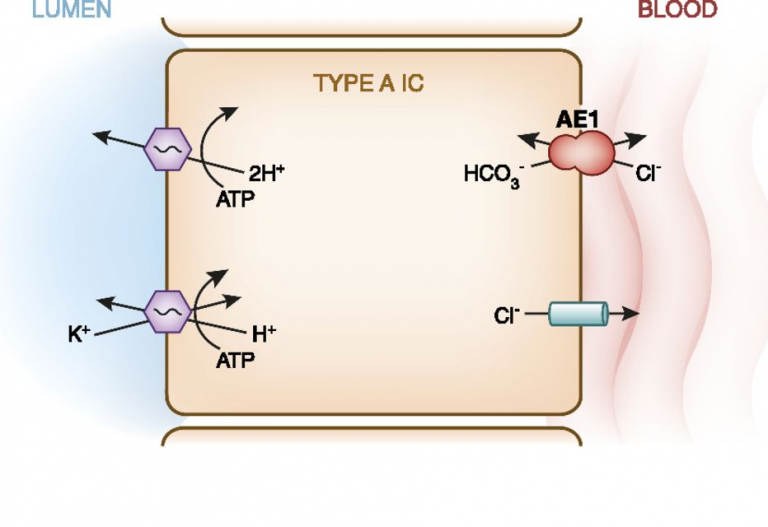
- Rest Type A intercalated cells of Collecting Duct
- Plasma HCO3– = 24mmol/L
- GFR = 180L/d
- ∴Filtered HCO3– = 4320 mmol/day
- HCO3– reabsorption is active
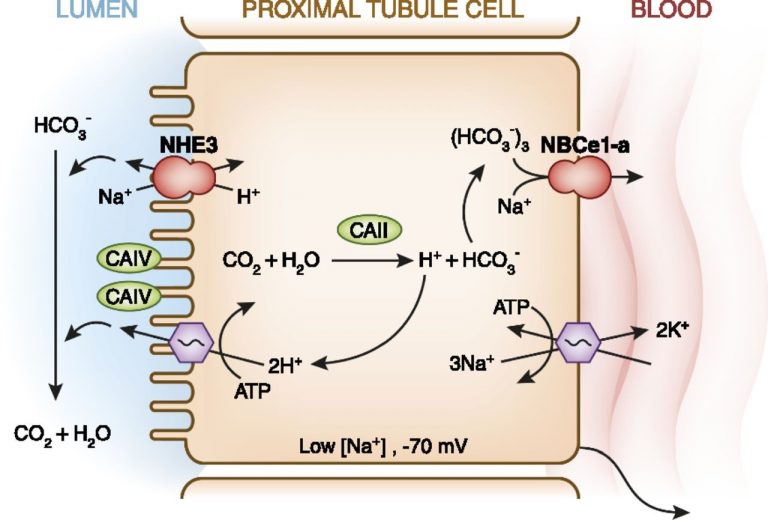
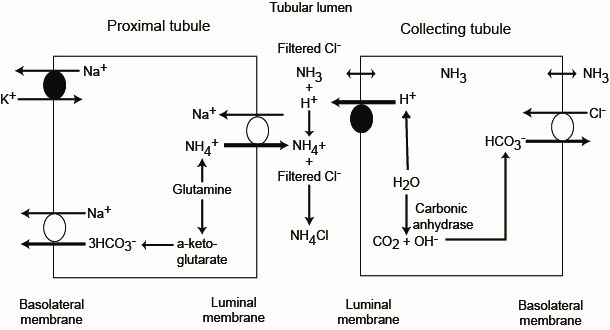
PCT
- CA present on apical membrane & inside tubule cells
- Catalyse: CO2 + H2O ⮂ H2CO3 ⮂ H+ + HCO3–
- HCO3– produced inside tubule cell is reabsorbed across BL membrane → peritubular caps
- H+ crosses apical membrane → CO2 + H2O
∴H+ is not added to urine → forms H2O with CA enzyme on apical membrane
Thick Ascending LoH
- Reabsorbs 15% HCO3– filtered
- Similar to PCT
DCT/Collecting Ducts
2) Fixed Acid Excretion
- Requires new HCO3– to be formed
- Difficult to excrete free H+ because its urine concentration is so low < 0.04mol/L even at the most acidic pH
- Fixed acid = 70 mmol/day
- 2 buffer system in URINE
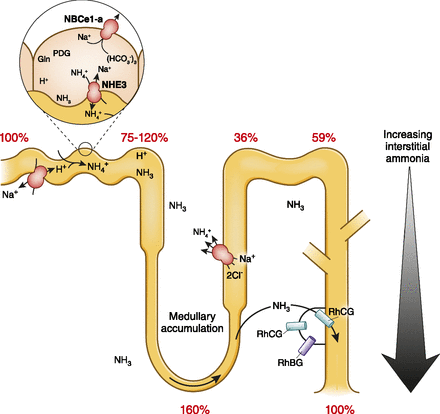
- AMMONIA → excretes 50 mol
- PHOSPHATE → excretes 20 mol
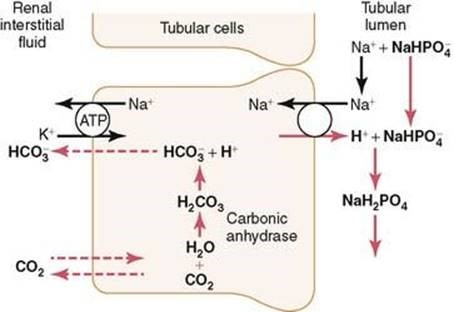
Phosphate buffer system
- H2PO4 (weak acid) ⮂ H+ + HPO42-
- H2PO4– is the “titratable acid” & refers to the amount of NaOH required to titrate the urine to pH 7.4 (which must equal the amount of H+ mol in urine)
- NH4+ is not part of titratable acidity. pKa 9.2
- ∴ exists as NH4+ & does not dissociate in urine
- ∴ doesn’t contribute to urinary pH (& ∴ not measured w titration)
- NB: To measure, you need to measure urinary NH4+
Note on Phosphate
- Plasma phosphate = 1 mmol/L
- 90% free, rest bound to plasma proteins
- GFR 180L / day ~160 mol /day filtered phosphate
- ~90% reabsorbed
- ∴ ~40 mol/day un-reabsorbed H2PO4– available for buffering
BUT
This system cannot be upregulated with ↑ acid load
- At pH 7.4 80% base HPO42- and 20% weak acid H2PO4–
- But as tubule fluid pH ↓, most is converted to acid H2PO4–
Collecting Ducts
- NH4+ secretion
- That which is reabsorbed in Thick Asc. Limb is put back into tubule & secreted
Overall : new HCO3– reabsorbed
Other buffers contributing to titratable acidity (but not that significant):
- Creatinine
- pKa 4.9 → at pH 4.4 in urine can contribute to titratable acidity
- B hydroxybutarate & acetoacetate
- pKa 4.5 → ketoacids appear in urine during DKA. Act as buffers during severe metabolic acidosis (because the H+ will only bind with them at low urinary pH due to their pKa)
- Author: Krisoula Zahariou