Eii / 24B11 / 17B14: Explain the mechanisms responsible for the cell resting membrane potential and explain the Gibbs Donnan Effect
24B11: Exam Report
(a) Explain the mechanisms responsible for the cell resting membrane potential (70% of marks). (b) Describe the Gibbs Donnan effect (30% of marks).
57% of candidates passed this question.
This question required a description of the separation of charge across the cell membrane due to selective ion permeabilities and concentrations.
The equilibrium voltage of an individual ion was best expressed using the Nernst Equation and of multiple ions by the Goldman-Hodgkin-Katz equation. Better responses were able to use the correct eponym, constants and/or logarithmic transformation (from the ln to log10).
The fundamental role of potassium conductance and the Na/K ATPase was also expected. The Gibbs-Donnan relationship was allocated 30% of marks so an explanation of the role of negatively charged intracellular proteins and the effect on ionic flux was expected.
17B14: Exam Report
Explain the mechanisms responsible for the cell resting membrane potential (60% of marks) and describe the Gibbs Donnan Effect (40% of marks).
35% of candidates passed this question.
A good answer included a definition of the resting membrane potential and a clear description of the factors that determine it. Explanation of these factors should have included a detailed description of the selective permeability of the membrane, electrochemical gradients and active transport mechanisms. Answers should demonstrate awareness of the Nernst equation and the Goldman-Hodgkin-Katz equation. These were often confused, sometimes with the Gibbs-Donnan effect. Descriptions of the Gibbs-Donnan effect generally lacked detail and understanding. The better answers included a definition and discussed in detail the influence of non-diffusible ions (intracellular proteins) on the distribution of diffusible ions.
Eii / 24B11 / 17B14: Explain the mechanisms responsible for the cell resting membrane potential (60 marks) and describe the Gibbs Donnan effect (40 marks)
Definition
The RMP is the electrical potential across a plasma membrane when the cell is in a non-excited state
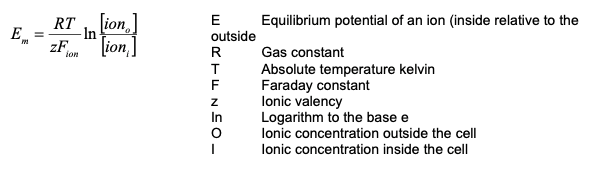
Equilibrium Potential
Nernst Equation
- The Nernst Potential (for each ion) calculates the potential difference the ion would produce if the membrane was permeable to it
Ion
K
[Intracellular] mM
150
[Extraceullular] mM
5
Nernst Potential (mV)
-90
Ion
Cl
[Intracellular] mM
10
[Extraceullular] mM
125
Nernst Potential (mV)
-70
Ion
Na
[Intracellular] mM
15
[Extraceullular] mM
150
Nernst Potential (mV)
+60
- At rest the Nernst potentials of Na & Cl most closely match Membrane Potential because these ions are freely diffusing, whereas Na is not
Goldman-Hodgkin-Katz Equation
- A modification of the Nernst Eqn
- Takes into account the permeability factors for each ion and constructs the total membrane resting potential
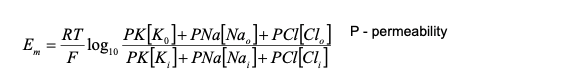
Resting Membrane Potential
- By definition the cell membrane must be semi-permeable (to create a potential)
- The cell membrane encompasses sodium (chemical gradient) and is freely permeable to K & Cl (electrochemical gradient)
- Ion channels and transport proteins regulate the movement of molecules across the membrane, allowing for an unequal distribution of charge through anions and cations
- The resulting chemical and electrical forces comprise the RMP
- The RMP varies depending on cell type
- Skeletal Muscle -90mV
- Myelinated Peripheral Nerve -70mV
- Cardiac Muscle -90mV
- Smooth Muscle -50mV
Generation of an Electro-Chemical Gradient
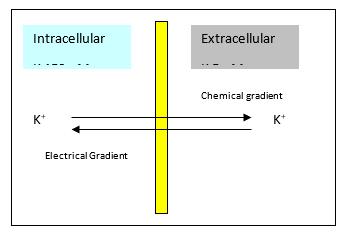
Potassium
- K ions cross the membrane through open channels
- Internal [] 150mM
- External [] 5mM
- K moves down its concentration gradient until opposing electrical gradient prevents further movement
- Results in an electrical potential
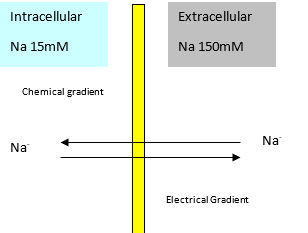
Sodium
- Sodium is not freely permeable
- Uneven concentration created by Na-K-ATPase
- Small electrochemical potential due to low membrane permeability
Chloride
- Chloride can pass freely
- Creates an electrochemical gradient
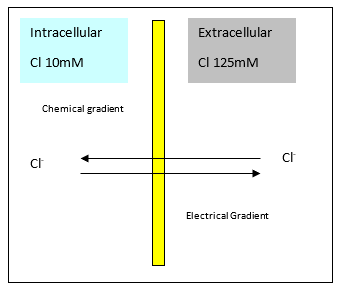
Gibbs-Donnan Effect
- Applies when one compartment contains non-diffusable ions
- Proteins which lack permeability are also contributing to the membrane potential
- The proteins inside the cell are adding a (small) negative charge to the overall membrane potential
- This accounts for the different plasma and ISF concentrations of the ions
Function of Membrane Potential
- Conduction of electrical signalling
- Excitation-Contraction-Coupling
- Electrochemical Gradients use energy to run cell machinery
- Author: Krisoula Zahariou