Bvi / 18A05: Compare and contrast the pharmacokinetic and pharmacodynamics of IV fentanyl and IV remifentanil (60 marks). Discuss the concept of context sensitive half-time using these drugs as examples (40 marks)
18A05: Exam Report
Compare and contrast the pharmacokinetic and pharmacodynamics of IV fentanyl and IV remifentanil (60 marks). Discuss the concept of context sensitive half-time using these drugs as examples (40 marks)
66% of candidates passed this question.
Well-constructed answers were presented in a table to compare pharmacokinetics and pharmacodynamics with a separate paragraph to discuss the concept of context sensitive half-time. Important pharmacokinetic points included: the differences in lipid solubility, ionised fractions and onset, and differences in metabolism. Marks were awarded for a definition of context-sensitive half-time. A discussion of these two drugs’ context-sensitive half-times should have included the differences in re-distribution into other compartments and rates of elimination.
Bvi / 18A05: Compare and contrast the pharmacokinetic and pharmacodynamics of IV fentanyl and IV remifentanil (60 marks). Discuss the concept of context sensitive half-time using these drugs as examples (40 marks)
Remifentanil
Fentanyl
Chemical
Remifentanil
Rapid onset, ultrashort acting
Synthetic selective µ agonist of PHENYLPERIDINE CLASS
Fentanyl
Synthetic opioid of PHENYLPIPERIDINE CLASS
Rapid acting, intermediate duration
PD
Remifentanil
CNS – analgesia no effect on ICP/IOP
CVS – ↓HR, ↓BP
MSK – muscle rigidity at high dose
Immuno – no histamine release
Resp – potent resp depression
Fentanyl
CNS – Analgesia
CVS – ↓HR more common cf. M
RESP – respiratory depression most marked of all opioids. POTENT ANTITUSSIVE & chest wall rigidity
GI – less N&V
IMMUNO –histamine release
PK
Remifentanil
A
IV administration only. 100% bioavailability
D
VD 0.4L/kg
Very small
Low lipid solubility
PPB 70%
M
Rapid ester hydrolysis
Non-specific plasma & tissue esterases
Into remifentanil acid x 5000 less potent cf. remi
E
Inactive metabolites
Renally excreted
Fentanyl
A
IV. 100% bioavailability
D
4L/kg
Larger cf. Remi
↑lipid solubility = rapid & extensive tissue penetration
PPB 80%
Tissue affinities
- Lungs → large, inactive storage site; 75% dose = pulmonary uptake
Fat & skeletal m. → redistribution sites
M
Liver
N-Demethylation
- To norfentanyl
- Less potent cf. F
High HER
∴depends on HBF
E
Norfentanyl excreted by kidneys
10% unchanged
(Given in renal failure as metabolites are not pharmacologically active)
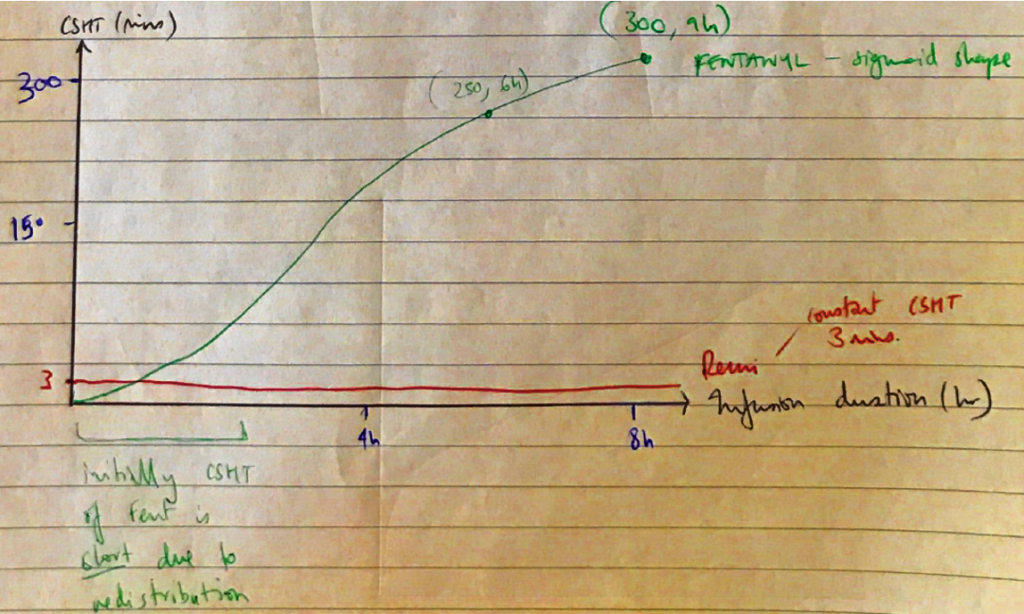
Context Sensitive Half Time
CSHT = the time taken for plasma [ ] to fall 50% following cessation of a drug infusion of specific duration
- “Context” refers to the duration
- Based on a multi-compartment model
- DETERMINANTS
- Duration of infusion
- Distribution to + from central/peripheral compartments
- Clearance
- Distinct from elimination half-life of a drug
- Elim t ½ = time taken for plasma [ ] of drug to fall by 50% during the elimination phase
- After a single bolus
- Based on Single Compartment Model
DETERMINANT: Clearance
\( \textbf{t ½ = } \frac{\text{ VD x 0.693}}{\text{Clearance}} \)
When SS is reached → CSHT = Elim t ½
@ SS; C5HT = Elim t ½
→ Otherwise CSHT is always less owing to redistribution (because it’s cleared & redistributed from central compartment)
CSHT of Remi v CSHT of Fentanyl
Remifentanil
Fentanyl
Cl
Remifentanil
High, Instant clearance
40 – 60ml/kg/min
Fentanyl
15mL/kg/min
VD
Remifentanil
0.4L/kg
Fentanyl
4L/kg
Elim t ½
Remifentanil
3 mins
Fentanyl
250 mins (6hr)
300 mins (9hr)
CSHT
Remifentanil
0.1 hrs – regardless of infusion duration
Fentanyl
5 hrs
Comments
Remifentanil
Remi has low lipid solubility + Low VD ∴ rapidly equilibrates with peripheral tissue → rapidly metabolised
Fentanyl
F = highly lipid soluble
∴rapid onset + large VD → basic drug, sequestered to pulmonary circulation
∴long CSHT
As duration of infusion exceeds 2hrs, it saturates the ‘inactive sites’(lungs, fat, skeletal m)
So when an infusion is terminated, the plasma [ ] cannot ↓ by redistributing to these sites
→ for plasma F to ↓ it must be eliminated by hepatic metabolism
- Author: Krisoula Zahariou